Advertisements
Advertisements
प्रश्न
Apply first law for an isobaric process.
उत्तर
The first law of thermodynamics for an isobaric process is given by ∆U = Q – P∆V
W = P∆V, ∆U = `"Q" - μ"RT"_"f" [1 - "T"_"i"/"T"_"f"]`
APPEARS IN
संबंधित प्रश्न
For work done to be reversible, the process should be ______
Draw a p-V diagram showing positive work at constant pressure.
Explain graphically (i) positive work with varying pressure, (ii) negative work with varying pressure, and (iii) positive work at constant pressure.
Explain work done during a thermodynamic process.
Draw the PV diagram for the isobaric process.
Explain in detail the isochoric process.
Among the amount of heat absorbed and the amount of work done by a system, ______
One mole of an ideal gas with `gamma` = 1.4 is adiabatically compressed so that its temperature rises from 27° C to 47° C. The change in the internal energy of the gas is (R = 8.3 J/mol.K) ____________.
Assertion: Equal volumes of monatomic and polyatomic gases are adiabatically compressed separately to equal compression ratio `("P"_2/"P"_1)`. Then monatomic gas will have greater final volume.
Reason: Among ideal gases, molecules of a monatomic gas have the smallest number of degrees of freedom.
Consider P-V diagram for an ideal gas shown in figure.
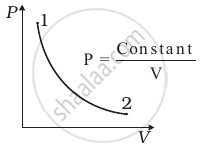
Out of the following diagrams (figure), which represents the T-P diagram?
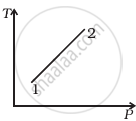 (i) |
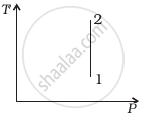 (ii) |
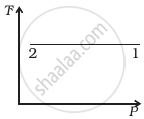 (iii) |
 (iv) |
