Advertisements
Advertisements
प्रश्न
Arrange the following:
In increasing order of basic strength: Aniline, p-nitroaniline and p-toluidine
उत्तर

In p-toluidine, the presence of electron-donating −CH3 group increases the electron density on the N-atom.
Thus, p-toluidine is more basic than aniline.
On the other hand, the presence of electron-withdrawing
−NO2 group decreases the electron density over the N−atom in p-nitroaniline. Thus, p-nitroaniline is less basic than aniline.
Hence, the increasing order of the basic strengths of the given compounds is as follows:
p-Nitroaniline < Aniline < p-Toluidine
APPEARS IN
संबंधित प्रश्न
Arrange the following:
Aniline, p-nitroaniline, p-methylaniline - in the increasing order of their basic strength
Arrange the following:
In increasing order of basic strength:
C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2
Arrange the following:
In decreasing order of basic strength in gas phase: C2H5NH2, (C2H5)2NH, (C2H5)3N and NH3
Write the structures of the main products of the following reactions:
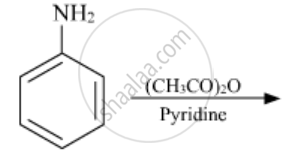
The following reaction takes place in the presence of:
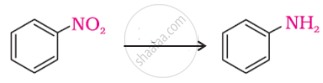
The most reactive amine towards dilute hydrochloric acid is:
The correct decreasing order of basic strength of the following species is ______.
\[\ce{H2O, NH3, OH-, NH^{-}2}\]
Account for the following:
Acylation of aniline is carried out in the presence of pyridine.
When ethanol is mixed with ammonia and passed over alumina the compound formed is which compound?
Which of the following is most basic?
By the presence of a halogen atom in the ring, what is the effect of this on basic property of aniline?
What is the correct decreasing order of the basic character of the three amines and ammonia?
Give reasons for the following observation:
pKb of aniline is lower than the m-nitroaniline.
Account for the following:
Arrange the following compounds in the increasing order of their basic strength in aqueous solution: CH3NH2,(CH3)3N,(CH3)2NH
Which of the following compound cannot be produced if 1-propane amine is treated with NaNO2 and HCl?
Arrange the decreasing order of pKb values.
\[\ce{C6H5NH2, C6H5NHCH3, C6H5CH2NH2, CH3NH2, NH3}\]
The correct order of the increasing basic nature of Ammonia, Methylamine and Aniline is:
