Advertisements
Advertisements
प्रश्न
Balance the following equations by the oxidation number method.
उत्तर
We can balance the given equation by oxidation number method-
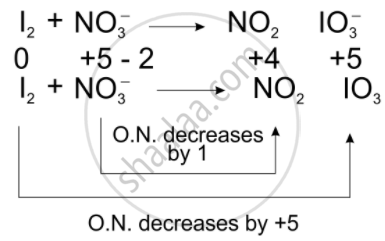
Total decrease in O.N. = 1
To equilize O.N. multiply
Balancing atoms other than
Balancing
APPEARS IN
संबंधित प्रश्न
Calculate the oxidation number of sulphur, chromium and nitrogen in H2SO5,
Balance the following redox equation by half-reaction method.
What is the change in oxidation number of Sulphur in following reaction?
Write balanced chemical equation for the following reactions:
Dichlorine heptaoxide
Balance the following equations by the oxidation number method.
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
Balance the following ionic equations.
In acidic medium, reaction,
