Advertisements
Advertisements
प्रश्न
Balance the given chemical reaction as per the instructions below.
\[\ce{NaOH + H2SO4 -> Na2SO4 + H2O}\]
Write the number of atoms of various elements in the reactants and products.
उत्तर
| Reactants (Left side) |
Products (Right side) |
|
| Element | Number of atoms | Number of atoms |
| Na | 1 | 2 |
| O | 5 | 5 |
| H | 3 | 2 |
| S | 1 | 1 |
APPEARS IN
संबंधित प्रश्न
Balance the chemical equation.
\[\ce{HNO3 +Ca(OH)2 -> Ca(NO3)2 + H2O}\]
Translate the following statement into chemical equation and then balance the equation:
Aluminium metal replaces iron from ferric oxide, Fe2O3, giving aluminium oxide and iron.
Translate the following statement into chemical equation and then balance the equation:
Barium chloride reacts with zinc sulphate to give zinc chloride and barium sulphate.
Balance the given equation:
HNO3 + Ca(OH)2  Ca(NO3)2 + H2O
Ca(NO3)2 + H2O
Potassium chlorate (KClO3) on heating forms potassium chloride and oxygen. Write a balanced equation for this reaction and indicate the evolution of gas.
What is meant by chemical reaction? Explain with the help of an example.
Give one example of a chemical reaction characterised by formation of a precipitate.
What do you understand by endothermic reactions?
You are given the solution of lead nitrate. In order to obtain a yellow precipitate you should mix with it a solution of:
(a) potassium chloride
(b) potassium nitride
(c) potassium sulphide
(d) potassium iodide
Write word equation for the following skeletal equation:
\[\ce{Zn + HCl -> ZnCl2 + H2}\]
Write your observation for the following chemical reaction and name the product formed :
When ammonium chloride is heated with sodium hydroxide.
Write symbolic representation for the following word equation and balance them :
Calcium oxide + Water → Calcium hydroxide
Write symbolic representation for the following word equation and balance them :
Aluminium + Chlorine → Aluminium chloride.
Write symbolic representation for the following word equation and balance them :
Iron + Sulphur → Iron sulphide.
Balance the following equation. Also name the products formed.
`"Mg" + "O"_2 → "MgO"`
Complete the following equation:
CH4 + O2 —>
A student weighed some raisins and recorded the weight as ‘x’. She then soaked the raisins in distilled water. After about 2 hours she removed the raisins, wiped them dry and weighed them again, and recorded that as ‘y’. The percentage of water absorbed by raisins may be determined using the relationship.
Explain the terms with examples.
Balanced equation
Show the formation of Na2O by the transfer of electrons.
Balance the following equation:
Zn + KOH → K2ZnO2 + H2
Balance the following equation:
Fe2O3 + CO → Fe + CO2
Sodium chloride reacts with silver nitrate to produce silver chloride and sodium nitrate
Write the equation.
Check whether it is balanced, if not balance it.
Find the weights of reactants and products.
State the law which this equation satisfies.
Match column A with column B.
| Column A | Column B |
| (a) Blue salt changes to white and then black | (i) Ammonium dichromate |
| (b) Orange coloured compound changes to green. | (ii) Iodine |
| (c) Red compound changes to brown and then yellow. | (iii) Zinc Nitrate |
| (d) White to yellow when hot and white when cold. | (iv) Copper sulphate |
| (e) Violet solid changes to violet vapours. | (v) Red Lead |
Answer the following question.
What is observed when 2 mL of dilute hydrochloric acid is added to 1 g of sodium carbonate taken in a clean and dry test tube? Write a chemical equation for the reaction involved.
Give one example in the case where supplying energy [given below] is necessary for a chemical reaction.
Pressure
Representation of the results of a chemical change – is a chemical equation.
For the equation: FeCl3 + 3NH4OH 3NH4Cl + Fe(OH)3 ↓
Answer the following:
Name the reactants and the products in the above equation.
Give reason for the following:
Silver salts are kept in dark coloured bottles.
Balance the following simple equation:
Al + N2 → AlN
Balance the following simple equation:
NO + O2 → NO2
Balance the following simple equation:
SO2 + O2 ⇌ SO3
Balance the following simple equation:
Al + H2SO4 → Al2(SO4)3 + H2
Balance the following simple equation:
\[\ce{N2 + H2 <=> NH3}\]
The following reaction is an example of a `4"NH"_3("g") + "SO"_2 -> 4"NO"("g") + 6"H"_2"O"("g")`
- displacement reaction
- combination reaction
- redox reaction
- neutralisation reaction
Which of the following are exothermic processes?
- Reaction of water with quick lime
- Dilution of an acid
- Evaporation of water
- Sublimation of camphor (crystals)
Give the characteristic tests for the following gases :
- CO2
- SO2
- O2
- H2
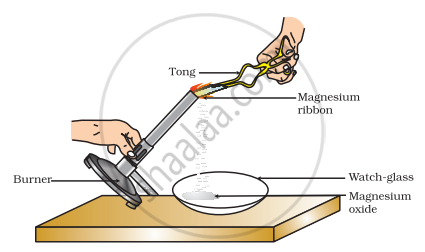
Which of the following is the correct observation of the reaction shown in the above set up?
To balance the following chemical equation the value of x and y should respectively be:
\[\ce{2NaOH + xAl_2O_3->yNaAlO_2 + H_2O}\]
