Advertisements
Advertisements
प्रश्न
Calculate the number of neutrons, protons and electrons:
- atomic number 3 and mass number 7
- atomic number 92 and mass number 238.
संख्यात्मक
उत्तर
- Number of neutrons – 4
Number of protons – 3
Number of electrons – 3 - Number of neutrons – 146
Number of protons – 92
Number of electrons – 92
shaalaa.com
क्या इस प्रश्न या उत्तर में कोई त्रुटि है?
APPEARS IN
संबंधित प्रश्न
Name the three sub-atomic particles of an atom.
Compare the properties of electrons, protons and neutrons.
Name the subatomic particle whose relative charge is : 0
What is a neutron? State its relative mass and charge.
Write down the number of neutrons in the nucleus of an atom having atomic number 17 and mass number 37.
State the number of neutrons in each of the atoms A to E. Also state which of the atoms A to E is a metal.
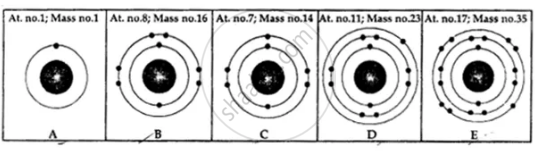
The number of neutrons present in 73Li is ______.
On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
James Chadwick discovered the fundamental particle called proton.
The hydrogen atom does not have ______.
