Advertisements
Advertisements
प्रश्न
Consider the following standard electrode potential values:
\[\ce{Sn^{2+}_{ (aq)} + 2e^- -> Sn_{(s)}}\]; E0 = −0.14 V
\[\ce{Fe^{3+}_{ (aq)} + e^- -> Fe^{2+}_{ (aq)}}\]; E0 = +0.77 V
What is the cell reaction and potential for the spontaneous reaction that occurs?
विकल्प
\[\ce{2Fe^{2+}_{ (aq)} + Sn^{2+}_{ (aq)} -> 2Fe^{3+}_{ (aq)} + Sn_{(s)}}\]; E0 = −0.91 V
\[\ce{2Fe^{3+}_{ (aq)} + Sn_{(s)} -> 2Fe^{2+}_{ (aq)} + Sn^{2+}_{ (aq)}}\]; E0 = +0.91 V
\[\ce{2Fe^{2+}_{ (aq)} + Sn^{2+}_{ (aq)} -> 2Fe^{3+}_{ (aq)} + Sn_{(s)}}\]; E0 = +0.91 V
\[\ce{2Fe^{3+}_{ (aq)} + Sn_{(s)} -> 2Fe^{3+}_{ (aq)} + Sn^{2+}_{ (aq)}}\]; E0 = +1.68 V
उत्तर
\[\ce{2Fe^{3+}_{ (aq)} + Sn_{(s)} -> 2Fe^{2+}_{ (aq)} + Sn^{2+}_{ (aq)}}\]; E0 = +0.91 V
Explanation:
Cell potential
\[\ce{E^0_{cell} = E^0_{cathode} - E^0_{anode}}\]
= +0.77 − (−0.14)
= +0.91 V
The cell reaction will be
\[\ce{2Fe^{3+}_{ (aq)} + Sn_{(s)} -> 2Fe^{2+}_{ (aq)} + Sn^{2+}_{ (aq)}}\]
APPEARS IN
संबंधित प्रश्न
The elements of 3d transition series are given as: Sc Ti V Cr Mn Fe Co
Answer the following: Which element has the highest m.p?
How would you account for the following:
Of the d4 species, Cr2+ is strongly reducing while manganese (III) is strongly oxidising.
Write the factors which are related to the colour of transition metal ions.
Explain why transition metals and their compounds act as a catalyst.
Why is \[\ce{HCl}\] not used to make the medium acidic in oxidation reactions of \[\ce{KMnO4}\] in acidic medium?
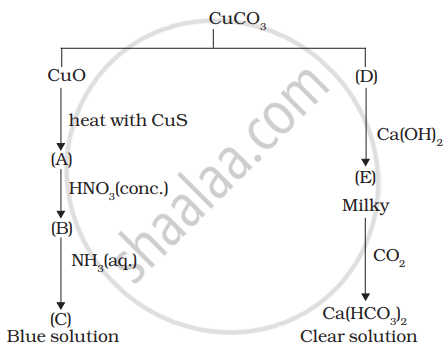
Identify A to E and also explain the reactions involved.
Mercury is the only metal liquid at room temperature due to its:-
Passing H2S gas into a mixture of Mn2+ and Ni2+, Cu2+, ions in an acidified aqueous solution precipitates.
Why are interstitial compounds well known for transition metals?
Describe the oxidising action of potassium dichromate and write the ionic equation for its reaction with iron (II) solution.
