Advertisements
Advertisements
प्रश्न
Define the term Co-ordination isomer.
उत्तर
Isomers which show interchange of ligands between cationic and anionic spheres of different metal ions are called co-ordination isomers.
संबंधित प्रश्न
Give one chemical test as an evidence to show that [Co (NH3)5Cl] are ionisation isomers.
Choose the most correct option.
Which of the following complexes exist as cis and trans isomers?
1. [Cr(NH3)2Cl4]-
2. [Co(NH3)5Br]2⊕
3. [PtCl2Br2]2- (square planar)
4. [FeCl2(NCS)2]2- (tetrahedral)
Answer the following in one or two sentences.
Predict whether the [Cr(en)2(H2O)2]3+ complex is chiral. Write the structure of its enantiomer.
Answer the following in one or two sentences.
Consider the complexes \[\ce{[Cu(NH3)4][PtCl4] and [Pt(NH3)4] [CuCl4]}\]. What type of isomerism these two complexes exhibit?
Answer the following question.
Draw geometric isomers and enantiomers of the following complex.
[Pt(en)3]4⊕
Answer the following question.
Draw geometric isomers and enantiomers of the following complex.
[Pt(en)2ClBr]2⊕
Which one of the following will give a pair of enantiomorphs?
Which kind of isomerism is possible for a complex [Co(NH3)4Br2]Cl?
Draw all possible geometrical isomers of the complex \[\ce{[Co(en)2Cl2]^+}\] and identify the optically active isomer.
What is linkage isomerism? Explain with an example.
Explain optical isomerism in coordination compounds with an example.
Identify the CORRECT statement about the following complex of platinum.
[PtCl2(en)2]2+
Which of the following is NOT an isomer of n-hexane?
The formula of two complexes X and Y of chromium are given below:
\[\ce{\underset{(X)}{[Cr(H2O)6]Cl3}}\] and \[\ce{\underset{(Y)}{[Cr(H2O)5Cl]Cl2.H2O}}\]
X and Y are examples of ____________ isomers.
What type of isomerism is present between (I) [Cr(H2O)6]Cl3 and (II) [Cr(H2O)5Cl]Cl2.H2O?
The relationship between compound (i) and (ii) is
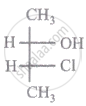 |
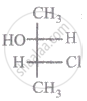 |
| (i) | (ii) |
The correct structure of Fe(CO)5 is
Draw geometric isomers of the following complex.
Geometrical isomers of Pt(NH3)2Cl2
Which of the following are isostructural pairs?
(A) \[\ce{SO^{2-}4}\] and \[\ce{CrO^{2-}4}\]
(B) SiCl4 and TiCl4
(C) NH3 and \[\ce{NO^-3}\]
(D) BCl3 and BrCl3
Which of the following compound is optically active?
The one that is not expected to show isomerism is ______.
Define Distereoisomers.
Match the pairs in column I (pairs of isomers) and column II (types of isomers)
| Column I (Pairs of isomers) |
Column II (Types of isomers) |
| (A) [Cr(H2O)5Cl]Cl2.H2O and [Cr(H2O)4Cl2]Cl.2H2O | (i) Ionization isomers |
| (B) [Co(en)2(NO2)2]+ and [Co(en)2(ONO2)]+ | (ii) Hydrate isomers |
| (C) [Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] | (iii) Linkage isomers |
| (D) [Pt(NH3)4Cl2] Br2 and [Pt(NH3)4Br2]Cl2 | (iv) Coordination isomers |
Draw the structure of cis isomers of Pt(NH3)2Cl2.
Draw the structure of trans isomers of Pt(NH3)2Cl2.
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Cr(H2O)5Cl]Cl2H2O and [Cr(H2O)4Cl2]Cl {.} 2H2O}\]
