Advertisements
Advertisements
प्रश्न
Which kind of isomerism is possible for a complex [Co(NH3)4Br2]Cl?
विकल्प
geometrical and ionization
geometrical and optical
optical and ionization
geometrical only
उत्तर
geometrical and ionization
APPEARS IN
संबंधित प्रश्न
Define the term Hydrated isomers.
Assertion: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason: Geometrical isomerism is not shown by complexes of coordination number 6.
The relationship between compound (i) and (ii) is
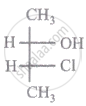 |
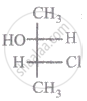 |
| (i) | (ii) |
Which of the following are isostructural pairs?
(A) \[\ce{SO^{2-}4}\] and \[\ce{CrO^{2-}4}\]
(B) SiCl4 and TiCl4
(C) NH3 and \[\ce{NO^-3}\]
(D) BCl3 and BrCl3
The one that is not expected to show isomerism is ______.
Which among the following solid is a non-polar solid?
Give cis isomer of [Co(NH3)4Cl2]⊕.
Draw the structure of cis isomers of Pt(NH3)2Cl2.
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Cr(H2O)5Cl]Cl2H2O and [Cr(H2O)4Cl2]Cl {.} 2H2O}\]
Three organic compounds A, B and C are non cyclic functional isomers of carbonyl compounds with molecular formula C4H8O. Isomers A and C give positive Tollen’s test while compound B does not give positive Tollen’s test but gives positive iodoform test. Compounds A and B on reduction with Zn amalgam and conc. HCl give the same product.
- Write the structures of the compounds A, B and C.
- Out of the compounds A, B and C, which one will be the least reactive towards addition of HCN.
