Advertisements
Advertisements
प्रश्न
Three organic compounds A, B and C are non cyclic functional isomers of carbonyl compounds with molecular formula C4H8O. Isomers A and C give positive Tollen’s test while compound B does not give positive Tollen’s test but gives positive iodoform test. Compounds A and B on reduction with Zn amalgam and conc. HCl give the same product.
- Write the structures of the compounds A, B and C.
- Out of the compounds A, B and C, which one will be the least reactive towards addition of HCN.
उत्तर
(a) [A] = CH3CH2CH2CHO
[B] = CH3COCH2CH3
[C] = CH3CH(CH3)CHO
(b) [B] will be least reactive towards addition of HCN.
APPEARS IN
संबंधित प्रश्न
Write the structural formula and IUPAC names of all possible isomers of the compound with molecular formula C3H8O.
Draw structure of cis isomer of [Co(NH3)4Cl2]+
Explain optical isomerism in coordination compounds with an example.
What is the number of moles of silver chloride precipitated when excess of aqueous silver nitrate is treated with [Co(NH3)4Cl2]Cl?
\[\ce{IUPAC}\] name of \[\ce{[Pt(NH3)2 Cl(NO2)]}\] is ______.
Assertion: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason: Geometrical isomerism is not shown by complexes of coordination number 6.
The relationship between compound (i) and (ii) is
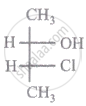 |
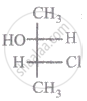 |
| (i) | (ii) |
Draw geometric isomers of the following complex.
Geometrical isomers of Pt(NH3)2Cl2
Draw the geometrical isomers of the following complexes [Co(NH3)4Cl2]+
Draw the structure of trans isomers of Pt(NH3)2Cl2.
