Advertisements
Advertisements
प्रश्न
Draw structure of cis isomer of [Co(NH3)4Cl2]+
उत्तर
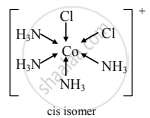
APPEARS IN
संबंधित प्रश्न
Answer the following in one or two sentences.
Predict whether the [Cr(en)2(H2O)2]3+ complex is chiral. Write the structure of its enantiomer.
Answer the following question.
Draw isomers of the following
[Cr(en2)Br2]⊕
Answer the following question.
Draw geometric isomers and enantiomers of the following complex.
[Pt(en)3]4⊕
Define the term Co-ordination isomer.
Draw the geometrical isomers of the following complexes [Pt(NH3)(H2O)Cl2].
Draw all possible geometrical isomers of the complex \[\ce{[Co(en)2Cl2]^+}\] and identify the optically active isomer.
Explain optical isomerism in coordination compounds with an example.
What are hydrate isomers? Explain with an example.
How many isomers are possible for an alkane having molecular formula C5H12?
The number of geometrical isomers of [CrCl2(en)2]+ is ____________.
Consider the two complexes given below:
\[\ce{\underset{(I)}{[Co(NH3)5SO4]Br}}\] and \[\ce{\underset{(II)}{[Co(NH3)5Br]SO4}}\]
I and II are ____________ isomers.
The formula of two complexes X and Y of chromium are given below:
\[\ce{\underset{(X)}{[Cr(H2O)6]Cl3}}\] and \[\ce{\underset{(Y)}{[Cr(H2O)5Cl]Cl2.H2O}}\]
X and Y are examples of ____________ isomers.
Which of the following has zero dipole moment?
Assertion: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason: Geometrical isomerism is not shown by complexes of coordination number 6.
Geometrical isomerism is not shown by
Which of the following compound show optical isomerism?
Which of the following shows maximum number of isomers?
Which of the following are isostructural pairs?
(A) \[\ce{SO^{2-}4}\] and \[\ce{CrO^{2-}4}\]
(B) SiCl4 and TiCl4
(C) NH3 and \[\ce{NO^-3}\]
(D) BCl3 and BrCl3
Which compound would exhibit optical isomers?
The number of geometrical isomers of \[\ce{[Co(NH3)3 (NO3)3]}\] are ______.
White precipitate of AgCl dissolves in aqueous ammonia solution due to formation of ______.
The one that is not expected to show isomerism is ______.
Give cis isomer of [Co(NH3)4Cl2]⊕.
Write structures for geometrical isomers of diamminebromochloroplatinum (II).
Explain the ionisation isomers.
Draw the geometrical isomers of the following complexes [Co(NH3)4Cl2]+
Give a chemical test to show that \[\ce{[Co(NH3)5Cl]SO4}\] and \[\ce{[Co(NH3)5SO4]CI}\] are ionisation isomers.
