Advertisements
Advertisements
प्रश्न
Draw the geometrical isomers of the following complexes [Pt(NH3)(H2O)Cl2].
उत्तर
Geometrical isomers of [Pt(NH3)(H2O)Cl2]:
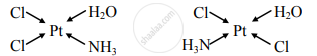
Cis isomer Trans isomer
संबंधित प्रश्न
Write the structural formula and IUPAC names of all possible isomers of the compound with molecular formula C3H8O.
Out of 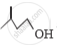 and
and 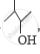 , which one is optically active and why ?
, which one is optically active and why ?
Why dextro and laevo rotatory isomers of Butan-2-ol are difficult to separate by fractional distillation?
Choose the most correct option.
Which of the following complexes exist as cis and trans isomers?
1. [Cr(NH3)2Cl4]-
2. [Co(NH3)5Br]2⊕
3. [PtCl2Br2]2- (square planar)
4. [FeCl2(NCS)2]2- (tetrahedral)
Answer in brief.
What are ionization isomers ? Give an example.
Answer the following question.
Draw isomers of the following
Ru(NH3)4Cl2
The pair [Co(NH3)5(SO4)]Br and [Co(NH3)5Br]SO4 exhibits ____________ isomerism
Which type of isomerism is exhibited by [Pt(NH3)2Cl2]?
Which one of the following pairs represents linkage isomers?
What is linkage isomerism? Explain with an example.
Which would exhibit coordination isomerism?
Identify the CORRECT statement about the following complex of platinum.
[PtCl2(en)2]2+
How many isomers are possible for an alkane having molecular formula C5H12?
Which of the following does NOT show optical isomerism?
____________ isomers are formed when the ligand has two different donor atoms.
How many donor groups are present in diethylene triamine?
What is the number of moles of silver chloride precipitated when excess of aqueous silver nitrate is treated with [Co(NH3)4Cl2]Cl?
Complex [COCl2(en)2]+ can
The number of geometrical isomers of \[\ce{[Co(NH3)3 (NO3)3]}\] are ______.
Which among the following solid is a non-polar solid?
Match the pairs in column I (pairs of isomers) and column II (types of isomers)
| Column I (Pairs of isomers) |
Column II (Types of isomers) |
| (A) [Cr(H2O)5Cl]Cl2.H2O and [Cr(H2O)4Cl2]Cl.2H2O | (i) Ionization isomers |
| (B) [Co(en)2(NO2)2]+ and [Co(en)2(ONO2)]+ | (ii) Hydrate isomers |
| (C) [Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] | (iii) Linkage isomers |
| (D) [Pt(NH3)4Cl2] Br2 and [Pt(NH3)4Br2]Cl2 | (iv) Coordination isomers |
Draw the geometrical isomers of the following complexes [Co(NH3)4Cl2]+
The co-ordination number of Co3+ ion in the complex [Co(NH3)4Cl2]⊕ is ______.
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Cr(H2O)5Cl]Cl2H2O and [Cr(H2O)4Cl2]Cl {.} 2H2O}\]
Which one of the following complex ions has geometrical isomers?
Three organic compounds A, B and C are non cyclic functional isomers of carbonyl compounds with molecular formula C4H8O. Isomers A and C give positive Tollen’s test while compound B does not give positive Tollen’s test but gives positive iodoform test. Compounds A and B on reduction with Zn amalgam and conc. HCl give the same product.
- Write the structures of the compounds A, B and C.
- Out of the compounds A, B and C, which one will be the least reactive towards addition of HCN.
