Advertisements
Advertisements
प्रश्न
Which kind of isomerism is possible for a complex [Co(NH3)4Br2]Cl?
पर्याय
geometrical and ionization
geometrical and optical
optical and ionization
geometrical only
उत्तर
geometrical and ionization
APPEARS IN
संबंधित प्रश्न
Answer in brief.
What are ionization isomers ? Give an example.
Draw all possible geometrical isomers of the complex \[\ce{[Co(en)2Cl2]^+}\] and identify the optically active isomer.
What is linkage isomerism? Explain with an example.
Which would exhibit coordination isomerism?
The formula of two complexes X and Y of chromium are given below:
\[\ce{\underset{(X)}{[Cr(H2O)6]Cl3}}\] and \[\ce{\underset{(Y)}{[Cr(H2O)5Cl]Cl2.H2O}}\]
X and Y are examples of ____________ isomers.
The relationship between compound (i) and (ii) is
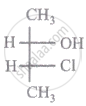 |
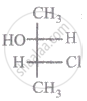 |
| (i) | (ii) |
Which of the following does not show optical isomerism?
Which of the following are isostructural pairs?
(A) \[\ce{SO^{2-}4}\] and \[\ce{CrO^{2-}4}\]
(B) SiCl4 and TiCl4
(C) NH3 and \[\ce{NO^-3}\]
(D) BCl3 and BrCl3
Indicate the type of isomerism exhibited by the following complex and draw the structures for this isomer:
\[\ce{[Pt(NH3)(H2O)Cl2]}\]
Match the pairs in column I (pairs of isomers) and column II (types of isomers)
| Column I (Pairs of isomers) |
Column II (Types of isomers) |
| (A) [Cr(H2O)5Cl]Cl2.H2O and [Cr(H2O)4Cl2]Cl.2H2O | (i) Ionization isomers |
| (B) [Co(en)2(NO2)2]+ and [Co(en)2(ONO2)]+ | (ii) Hydrate isomers |
| (C) [Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] | (iii) Linkage isomers |
| (D) [Pt(NH3)4Cl2] Br2 and [Pt(NH3)4Br2]Cl2 | (iv) Coordination isomers |
