Advertisements
Advertisements
प्रश्न
Draw an electron dot diagram for the formation of the following. State the type of bonding present in them.
Hydroxyl ion
उत्तर
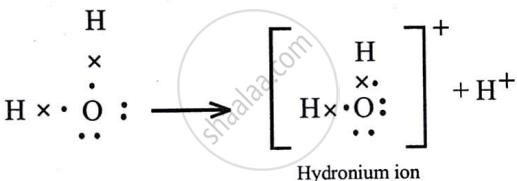
It has polar covalent bond.
APPEARS IN
संबंधित प्रश्न
Elements forming ionic compounds attain noble gas configuration by either gaining or losing electrons from their outermost shells. Give reason to explain why carbon cannot attain noble gas configuration in this manner to form its compounds.
Choose the correct answer from the options given below:
Which of the following is a common characteristic of a covalent compound?
1) high melting point
2) consists of molecules
3) always soluble in water
4) Conducts electricity when it is in the molten state
Why is graphite used for making dry cell electrodes but diamond is not?
Describe the structure of graphite with the help of a labelled diagram.
Two non-metals combine with each other by the sharing of electrons to form a compound X.
(a) What type of chemical bond is present in X?
(b) State whether X will have a high melting point or low melting point.
(c) Will it be a good conductor of electricity or not?
(d) Will it dissolve in an organic solvent or not?
The pencil leads are made of mainly:
(a) lithium
(b) charcoal
(c) lead
(d) graphite
Compare the compounds carbon tetrachloride and sodium chloride with regard to solubility in water and electrical conductivity.
Element A has 2 electrons in its M shell. Element B has atomic number 7.
Write equations to show how A and B form ions.
Name gas with rotten egg smell.
Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g., hydrogen. After the formation of four bonds, carbon attains the electronic configuration of ______.
