Advertisements
Advertisements
प्रश्न
Enlist the properties of glucose that can not be explained on the basis of open chain structure of it
उत्तर
Although the open chain structure of D (+) − Glucose explains most of its reactions, it fails to explain the following facts:
a) D (+)-Glucose does not undergo certain characteristic reactions of aldehydes
eg., Glucose does not form NaHSO3 addition product, aldehyde-ammonia adduct 2, 4 − DNP derivative and does not respond to Schiff’s reagent test.
b) Glucose reacts with NH2OH to form an oxime but glucose pentaacetate does not react
with NH2OH, which implies that the free aldehyde group is absent in glucose pentaacetate
c) D(+)-Glucose exists in two stereoisomeric crystalline forms, i.e. α-glucose and
β-glucose, called anomers. α-D(+)-Glucose is obtained when a concentrated aqueous or
alcoholic solution is crystallised at 303 K. It has a melting point of 419 K and has a
specific rotation of +111° in a freshly prepared aqueous solution.
However, when glucose is crystallised from water above 371 K, β-D(+)-glucose is
obtained. It has a melting point of 423 K and has a specific rotation of +19.2° in a
freshly prepared aqueous solution. This behaviour could not be explained by the open
chain structure of glucose.
APPEARS IN
संबंधित प्रश्न
Write the reaction that indicates the presence of -CHO group in glucose
Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Glucose on reaction with HI gives n-hexane. What does it suggest about the structure of glucose?
What do you observe when glucose solution is heated with Tollen’s reagent?
Fill in the blanks by choosing the appropriate word/words from those given in the brackets:
(iodoform, acetaldehyde, positive, greater, acidic, acetone, disaccharide, negative, increases, glucose, decreases, chloroform, polysaccharide, lactose, lesser, basic, cationic hydrolysis, anionic hydrolysis)
Sucrose is a _________ and yields upon hydrolysis, a mixture of ________ and fructose.
What do you observe when glucose is treated with bromine water?
Write the reactions involved when D-glucose is treated with the following reagent:
Br2 water
The spatial arrangement of the given molecule is denoted by:

Acetylation of glucose yields ____________.
Glucose does not give Schiff’s test because of the formation of cyclic ____________.
The symbols D and L represents ____________.
Glucose is found to exist in two different α and β crystalline forms. These forms can be obtained by:
(i) The α form of glucose is obtained by crystallisation from a concentrated solution of glucose at 303 K.
(ii) The β form of glucose is obtained by crystallisation from a concentrated solution of glucose at 303 K.
(iii) The β form is obtained by crystallisation from hot and saturated aqueous solution at 371 K.
(iv) The α form is obtained by crystallisation from hot and saturated aqueous solution at 371 K.
The number of chiral carbon atoms present in cyclic structure α-D(+) glucose:
The α-D glucose and β-D glucose differ from each other due to difference in carbon atom with respect to its ____________.
The number of chiral carbons in ß-D(+) glucose is ____________.
Which one of the following reactions is not explained by the open chain Structure of glucose?
In the following reaction, identify A and B:
\[\begin{array}{cc}
\ce{C6H12O6 ->[Acetic anhydride] A}\\
\downarrow \text{Conc. nitric acid}\phantom{...}\\
\ce{B}\phantom{.................}\end{array}\]
Which of the following pairs represents anomers?
Three structures are given below in which two glucose units are linked. Which of these linkages between glucose units are between C1 and C4 and which linkages are between C1 and C6?
| (I) |  |
| (II) | 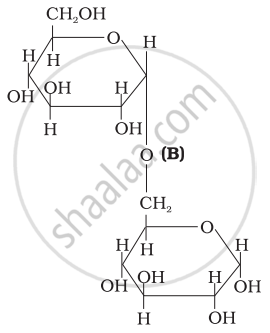 |
| (III) | 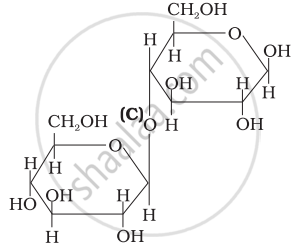 |
How will you distinguish 1° and 2° hydroxyl groups present in glucose? Explain with reactions.
Assertion: D (+) – Glucose is dextrorotatory in nature.
Reason: ‘D’ represents its dextrorotatory nature.
Account for the following:
There are 5 OH groups in glucose
Glucose with excess of phenyl hydrazine forms ______.
The number of asymmetric carbon atoms in the glucose molecule in open and cyclic form is ______.
Give the reaction of glucose with hydrogen cyanide. Presence of which group is confirmed by this reaction?
Give a reason for the following observations:
Penta-acetate of glucose does not react with hydroxylamine.
