Advertisements
Advertisements
प्रश्न
Ethanol can be oxidized to ethanoic acid. Write the equation and name the oxidizing agent.
उत्तर
Ethanol is oxidised to acetic acid.
\[\ce{C2H5OH + 2[O] ->[Acidified][K2Cr2O7] \underset{Acetic acid}{CH3COOH} + H2O}\]
The oxidising agent is Potassium dichromate, or Ethanoic acid.
APPEARS IN
संबंधित प्रश्न
In order to study saponification reaction, we first prepare 20% solution of sodium hydroxide. If we record the temperature of this solution just after adding sodium hydroxide flakes to water and also test its nature using litmus, it may be concluded that the process of making this solution is
(A) exothermic and the solution is alkaline
(B) endothermic and the solution is alkaline
(C) endothermic and the solution is acidic
(D) exothermic and the solution is acidic
What is the common name of methanol?
Choose those compounds from the following which can turn blue litmus solution red:
HCHO, CH3COOH, CH3OH, C2H5OH, HCOOH, CH3CHO
Give reasons for your choice.
Consider the following organic compounds:
HCHO, C2H5OH, C2H6, CH3COOH, C2H5CI
Choose two compounds which can react in the presence of conc. H2SO4 to form an ester. Give the name and formula of the ester formed.
Write the name of the first three members of the carboxylic acid series.
Acetic acid is a typical acid. Write one equation in case of its reactions with a metal?
What is thr boilng point of acetic acid?
State the observation
When the gaseous product obtained by dehydration of ethyl alcohol is passed through bromine water.
Ester is formed by the reaction between ______.
The reaction between acetic acid and sodium carbonate is shown in the following figure.
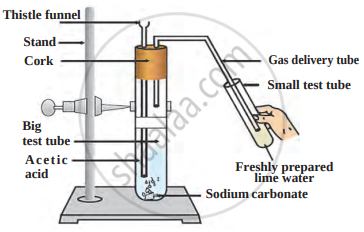
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
