Advertisements
Advertisements
प्रश्न
Expansion of a gas in vacuum is called free expansion. Calculate the work done and the change in internal energy when 1 litre of ideal gas expands isothermally into vacuum until its total volume is 5 litre?
उत्तर
During free expansion, external pressure is zero, so Work done, w = – pextΔV
= – 0(5 – 1) = 0
Since the gas is expanding isothermally, therefore, q = 0
ΔU = q + w = 0 + 0 = 0
APPEARS IN
संबंधित प्रश्न
The pressure-volume work for an ideal gas can be calculated by using the expression w = `- int_(v_i)^(v_f) p_(ex) dV`. The work can also be calculated from the pV– plot by using the area under the curve within the specified limits. When an ideal gas is compressed (a) reversibly or (b) irreversibly from volume Vi to Vf. choose the correct option.
How will you calculate work done on an ideal gas in a compression, when change in pressure is carried out in infinite steps?
Represent the potential energy/enthalpy change in the following processes graphically.
(a) Throwing a stone from the ground to roof.
(b) \[\ce{1/2 H2(g) + 1/2 Cl2 (g) ⇌ HCl (g) Δ_rH^Θ = - 92.32 kJ mol^{-1}}\]
In which of the processes potential energy/enthalpy change is contributing factor to the spontaneity?
1.0 mol of a monoatomic ideal gas is expanded from state (1) to state (2) as shown in figure. Calculate the work done for the expansion of gas from state (1) to state (2) at 298 K.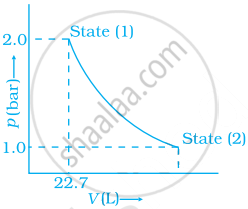
An ideal gas is allowed to expand against a constant pressure of 2 bar from 10 L to 50 L in one step. Calculate the amount of work done by the gas. If the same expansion were carried out reversibly, will the work done be higher or lower than the earlier case? (Given that 1 L bar = 100 J)
Match the following :
| A | B |
| (i) Adiabatic process | (a) Heat |
| (ii) Isolated system | (b) At constant volume |
| (iii) Isothermal change | (c) First law of thermodynamics |
| (iv) Path function | (d) No exchange of energy and matter |
| (v) State function | (e) No transfer of heat |
| (vi) ΔU = q | (f) Constant temperature |
| (vii) Law of conservation of energy | (g) Internal energy |
| (viii) Reversible process | (h) Pext = o |
| (ix) Free expansion | (i) At constant pressure |
| (x) ΔH = q | (j) Infinitely slow process which proceeds through a series of equilibrium states. |
| (xi) Intensive property | (k) Entropy |
| (xii) Extensive property | (l) Pressure |
| (m) Specific heat |
For silver Cp (J K-1 mol-1) = 23 + 0.01 T. If the temperature (T) of 3 moles of silver is raised from 300 K to 1000 K at 1 atom pressure, the value of ΔH will be close to ______.
Find the work done when 2 moles of hydrogen expand isothermally from 15 to 50 litres against a constant pressure of 1 atm at 25°C.
Five moles of an ideal gas at 1 bar and 298 K is expanded into the vacuum to double the volume. The work done is ______.
An ideal gas expands in volume from 1 × 10−3 to 1 × 10−2 m3 at 300 K against a constant pressure of 1 × 105 Nm−2. The work done is ______.
