Advertisements
Advertisements
प्रश्न
Explain solid solutions of metals and vacancy through aliovalent cations.
उत्तर
- Solid solutions of metals (alloys):
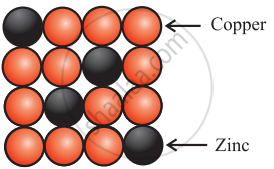
Brass
Brass is an alloy of Cu and Zn. In brass, host Cu atoms are replaced by impurity of Zn atoms. The Zn atoms occupy regular sites of Cu atoms as shown in the figure. - Formation of vacancy through aliovalent impurity:
Vacancies are created by the addition of impurities of aliovalent ions (that is, ions with oxidation state different from that of host ions) to an ionic solid.
e.g. Consider a small amount of SrCl2 impurity added to NaCl during its crystallization. The added Sr2+ ions (O.S. = +2) occupy some of the regular sites of Na+ host ions (O.S. = +1). In order to maintain electrical neutrality, every Sr2+ ion removes two Na+ ions. One of the vacant lattice sites created by removal of two Na+ ions is occupied by one Sr2+ ion. The other site of Na+ ion remains vacant as shown in the figure.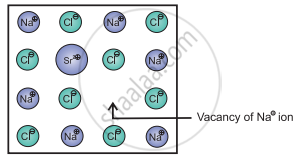
Vacancy through aliovalent ion
APPEARS IN
संबंधित प्रश्न
What are the consequences of Schottky defect?
Explain with diagram, the vacancy defect.
Give the classification of stoichiometric point defects.
What is a substitutional impurity defect?
Which of the following is an example of substitutional impurity defect?
Which among the following solids shows Frenkel defect?
Which of the following match is CORRECT?
(I) Frenkel defect: Electrical neutrality of the compound is preserved.
(II) Schottky defect: Density of the substance decreases.
(III) Schottky defect: Combination of vacancy defect and interstitial defect.
(IV) Frenkel defect: Small difference between size of cation and anion.
Which among the following statements is true about Schottky defect?
Which of the following pair of ionic crystals show Schottky defect?
Schottky defect in crystals is observed when ____________.
Schottky defect is formed in ionic compounds with ____________.
Write the consequences of Schottky defect with reasons.
Explain metal deficiency defect with examples.
In which among the following solids, Schottky defect is not observed?
When electrons are trapped into the crystal in anion vacancy, the defect is known as ______.
Explain the following term:
Substitutional impurity defect
Explain the following term:
Interstitial impurity defect
Explain Self interstitial defect in elemental solid.
Explain the interstitial defect.
What is a vacancy defect?
