Advertisements
Advertisements
प्रश्न
Explain, why [Co(NH3)6]3+ ion is low spin? Calculate number of unpaired electrons and write the geometry of [Co(NH3)6]3+.
उत्तर
NH3 is a strong field ligand. Strong field ligands cause larger splitting of d orbitals and pairing of electrons is favoured. Hence, [Co(NH3)6]3+ is a low spin complex.
- In [Co(NH3)6]3+ ion, oxidation state of cobalt is +3.
Valence shell electronic configuration of Co3+ is:
- Number of ammine ligands is 6. Therefore, the number of vacant metal ion orbitals required for bonding with ligands must be six. Complex is low spin, so the pairing of electrons will take place prior to hybridisation.
Electronic configuration after pairing would be:
- Six orbitals available for hybridisation are two 3d, one 4s, three 4p orbitals.
The orbitals for hybridization are decided from the number of ammine ligands which is six. Here, (n−1)d orbitals participate in hybridization since it is the low spin complex. - Electronic configuration after complex formation is:
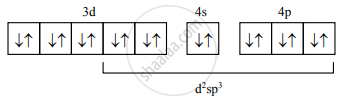
- Number of unpaired electrons = 0
- Geometry of the complex ion = Octahedral
APPEARS IN
संबंधित प्रश्न
Answer in brief.
What are the high-spin and low-spin complexes?
Answer in brief.
[CoCl4]2- is a tetrahedral complex. Draw its box orbital diagram. State which orbitals participate in hybridization.
Answer in brief.
What are strong field and weak field ligands ? Give one example of each.
Answer in brief.
With the help of the crystal field, the energy-level diagram explains why the complex [Cr(en)3]3⊕ is coloured.
Answer the following question.
Give valence bond description for the bonding in the complex [VCl4]-. Draw box diagrams for the free metal ion. Which hybrid orbitals are used by the metal? State the number of unpaired electrons.
Answer the following question.
Draw a qualitatively energy-level diagram showing d-orbital splitting in the octahedral environment. Predict the number of unpaired electrons in the complex [Fe(CN)6]4-. Is the complex diamagnetic or paramagnetic? Is it coloured? Explain.
Identify the number of donor groups present in EDTA.
The number of unpaired electrons in the complex ion [NiCl4]2− is ____________.
Identify the CORRECT statements regarding [Co(NH3)6]3+.
I. Oxidation state of metal ion = +3
II. It is a high spin complex.
III. It is paramagnetic.
IV. Metal ion undergoes d2sp3 hybridization.
Which one of the following complexes can exhibit geometrical isomerism?
What is the type of magnetic behavior and geometry respectively in Cuproammonium sulphate (Atomic number of Cu = 29)?
Identify the increasing order of effective magnetic moment of the following elements in their +2 oxidation state.
[Fe (Z = 26), Co (Z = 27), Ni (Z = 28), Cu (Z = 29)]
Explain the formation of [CoF6]3Θ complex with respect to
- Hybridisation
- Magnetic properties
- Inner/outer complex
- Geometry
Describe the bonding in the tetrahedral complex Ni(CO)4 on the basis of valence bond theory. Give the orbital diagrams of metal atoms in free state and in the complex. Mention the number of unpaired electrons in the complex.
A compound forms hcp structure. What is the number of (a) octahedral voids, (b) tetrahedral voids, (c) total voids formed in 0.2 mol of the compound?
A compound forms a hep structure. Calculate the number of octahedral voids in 0.4 mol. (NA = 6.022 × 1023 )
Give the limitations of VBT.
A compound forms hexagonal close packed (hcp) structure. What is the number of (i) Octahedral voids, (ii) Tetrahedral voids, (iii) Total voids formed in 0.7 mol of it.
Mention the type of hybridization in [Co(NH3)6]3+ complex.
Mention the number of unpaired electrons and geometry of the following complex:
\[\ce{[Ni(Cl)4]^{2-}}\]
Mention the number of unpaired electrons and geometry of the following complex:
\[\ce{[Ni(CN)4]^2-}\]
