Advertisements
Advertisements
प्रश्न
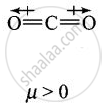
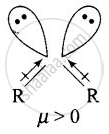
Explain why is \[\ce{O = C =O}\] nonpolar while \[\ce{R - O - R}\] is polar.
उत्तर
\[\ce{O = C =O}\] molecule is linear so that the polarities of two C – O bonds get cancelled and the molecule is linear.
Ethers have structures similar to water and have angular or bent structure. Therefore, the polarity of two R – O groups does not get cancelled and these have net dipole moment. Thus, \[\ce{R - O - R}\] is polar.
APPEARS IN
संबंधित प्रश्न
\[\ce{CH3 - CH = CH2 ->[H2O/H^+]}\]
Write the structure of the product of the following reaction:
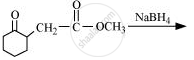
Write the structure of the compound whose IUPAC name is as follows:
1-Phenylpropan-2-ol
Write the structure of the compound whose IUPAC name is as follows:
2, 3-Diethylphenol
\[\ce{CH3-O-CH(CH3)2 + HI ->}\] Products is/are:
Which one of the following are correctly arranged on the basis of the property indicated?
What is the structure and IUPAC name of glycerol?
Epoxides are
Cresol is:-
Ether can be used
