Advertisements
Advertisements
Question
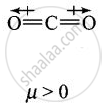
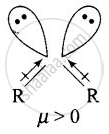
Explain why is \[\ce{O = C =O}\] nonpolar while \[\ce{R - O - R}\] is polar.
Solution
\[\ce{O = C =O}\] molecule is linear so that the polarities of two C – O bonds get cancelled and the molecule is linear.
Ethers have structures similar to water and have angular or bent structure. Therefore, the polarity of two R – O groups does not get cancelled and these have net dipole moment. Thus, \[\ce{R - O - R}\] is polar.
APPEARS IN
RELATED QUESTIONS
Write the structure of the compound whose IUPAC name is as follows:
3, 5-Dimethylhexane−1, 3, 5-triol
Write the structure of the compound whose IUPAC name is as follows:
1-Ethoxypropane
Write the structure of the compound whose IUPAC name is as follows:
2-Ethoxy-3-methylpentane
Write the structure of the compound whose IUPAC name is as follows:
Cyclohexylmethanol
Write the structure of the compound whose IUPAC name is as follows:
3-Cyclohexylpentan-3-ol
\[\ce{CH3-O-CH(CH3)2 + HI ->}\] Products is/are:
Write the structures of the isomers of alcohols with molecular formula \[\ce{C4H10O}\]. Which of these exhibits optical activity?
Which of the following does not form phenol or peroxide?
Epoxides are
Write the structure of the compound whose IUPAC name is as follows:
4-Chloro-3-ethylbutan-1-ol
