Advertisements
Advertisements
प्रश्न
How do we obtain characteristic x-ray spectra?
उत्तर
Characteristic x-ray spectra:
1. X-ray spectra snow some narrow peaks at some, well-defined wavelengths when the target is hit by fast electrons. The line spectrum showing these peaks is called the characteristic x-ray spectrum.
2. When an energetic electron removes electrons from K-shell electrons than the electrons from the outer orbit jump to fill the vacancy.
3. Energy difference between the levels is given out as x-ray photons of definite wavelength.
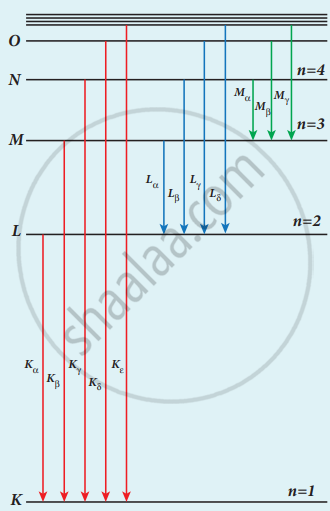
Origin of characteristic x-ray spectra
K-Series (Kα and Kβ): This line is due to electronic transitions from L, M, and N shells to K-level.
L-Series (Lα and Lβ): It arises due to electronic transitions from M, N, and O shells.
APPEARS IN
संबंधित प्रश्न
Why we do not see the wave properties of a baseball?
A proton and an electron have the same kinetic energy. Which one has a greater de Broglie wavelength? Justify.
Write the relationship of de Broglie wavelength λ associated with a particle of mass m in terms of its kinetic energy K.
An electron and an alpha particle have the same kinetic energy. How are the de Broglie wavelengths associated with them related?
What is Bremsstrahlung?
Describe briefly Davisson – Germer experiment which demonstrated the wave nature of electrons.
What should be the velocity of the electron so that its momentum equals that of 4000 Å wavelength photon.
Calculate the de Broglie wavelength of a proton whose kinetic energy is equal to 81.9 × 10–15 J.
(Given: mass of proton is 1836 times that of electron).
An electron is accelerated through a potential difference of 81 V. What is the de Broglie wavelength associated with it? To which part of the electromagnetic spectrum does this wavelength correspond?
The ratio between the de Broglie wavelength associated with proton accelerated through a potential of 512 V and that of alpha particle accelerated through a potential of X volts is found to be one. Find the value of X.
