Advertisements
Advertisements
प्रश्न
How will you bring about the following conversions?
2-methyl propan-2-ol to 2-methylpropene
उत्तर
2-Methylpropan-2-ol undergoes dehydration when passed over hot copper at 573 K.
\[\begin{array}{cc}
\ce{H3C - C(OH) - CH3 ->[Cu/573K][dehydration] H3C - C = CH2}\\
\phantom{}|\phantom{...............................}|\phantom{.}\\
\phantom{....}\ce{\underset{\text{2-Methylpropan-2-ol}}{CH3}\phantom{..................}\underset{\text{2-Methylpropene}}{CH3}}\phantom{....}
\end{array}\]
संबंधित प्रश्न
Give the reagents and conditions necessary to prepare phenol from Chlorobenzene.
Arrange the following in decreasing order of acid strength.
CH3OH, CH3–CH2–OH, CH3–CH(OH)–CH3, (CH3)3–C–OH
With the help of chemical equations show what happens when cumene hydroperoxide is treated with dil. acid.
How will you bring about the following conversions?
isopropyl alcohol to acetone
An organic compound gives hydrogen on reaction with sodium metal. It forms an aldehyde with molecular formula C2H4O on oxidation with pyridinium chlorochromate. Give the chemical equations in support of these observations.
In the Lucas test for alcohols, the appearance of turbidity is due to the formation of ____________.
Which of the following compounds does not react with bromine in alkaline medium?
α-butylene when subjected to hydroboration oxidation reaction, yields ______.
Sodium metal with ethyl alcohol gives __________ gas.
Bromination of phenol, will NOT give:
Propane when treated with cold cone. H2SO4 forms a compound which on heating with water gives ______.
Which of following elements does not react with hot concentrated sulphuric acid?
Phenoxide ion is more stable than phenol due to the ____________.
Which of the following alcohols has tertiary allylic carbon?
\[\ce{Isopropyl alcohol + acidic K2Cr2O7 -> X}\]
Identify product 'X' in the above reactions.
Which of the following conversion explains the acidic nature of alcohols?
The number of σ bonds in carbolic acid is ______.
Identify product B in the following conversion?
\[\ce{Phenol ->[NaOH] A ->[CH3I] B}\]
Which of the following alcohols is NOT prepared by reduction of carbonyl compounds?
Identify the product X in the following reaction.
\[\ce{Phenol ->[Na2Cr2O7][H2SO4] X}\]
Identify the compound amongst the following of which 0.1 M aqueous solution has highest boiling point.
Amongst the following alcohols which would react fastest with cone. HCl and ZnCl2?
What is the product formed when aniline is treated with \[\ce{NaNO2 + HCl}\] previous to hydrolysis?
The product C in the following reaction is

The major product obtained in the following reaction is

Arrange O - H, C - H and N - H bonds in increasing order of their bond polarity.
Arrange the following compounds in an increasing order of their solubility in water:
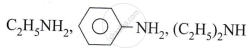
Explain: Phenols are acid while alcohol is neutral.
