Advertisements
Advertisements
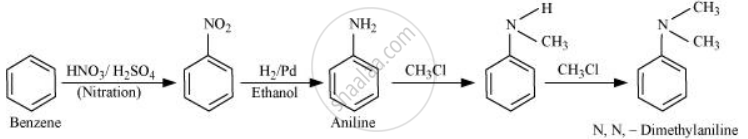
प्रश्न
How will you convert Benzene into N, N-dimethylaniline?
उत्तर
APPEARS IN
संबंधित प्रश्न
Give reasons for the following: (CH3)2NH is more basic than (CH3)3N in an aqueous solution.
Give reason for the following:
Primary amines have higher boiling point than tertiary amines.
Arrange the following: C2H5NH2, C2H5OH, (CH3)3N – in the increasing order of their boiling point
- Write structures of different isomeric amines corresponding to the molecular formula C4H11N.
- Write the IUPAC names of all the isomers.
- What type of isomerism is exhibited by different pairs of amines?
Complete the following acid-base reaction and name the product:
\[\ce{CH3CH2CH2NH2 + HCl ->}\]
Account for the following:
Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Arrange the following:
In increasing order of boiling point:
C2H5OH, (CH3)2NH, C2H5NH2
Accomplish the following conversion:
Aniline to p-bromoaniline
Complete the following reaction:
\[\ce{C6H5N2Cl + H3PO2 + H2O ->}\]
Arrange the following in the increasing order of their pKb values:
C6H5NH2, C2H5NH2, C6H5NHCH3
Choose the most correct option.
Which one of the following compounds has the highest boiling point?
The CORRECT decreasing order of boiling points is:
Among the following isomeric amines, an amine having highest boiling point is:
A compound Z with molecular formula \[\ce{C3H9N}\] reacts with \[\ce{C6H5SO2Cl}\] to give a solid, insoluble in alkali. Identify Z.
Assertion: N-Ethylbenzene sulphonamide is soluble in alkali.
Reason: Hydrogen attached to nitrogen in sulphonamide is strongly acidic.
Acetic acid exist as dimer in benzene due to
Dimerisation of carboxylic acids is due to
Which of the following amines form maximum hydrogen bonds within themselves?
Arrange the decreasing boiling point.
\[\ce{CH3COOH, C2H5OH, CH3NH2, CH3OCH3}\]
Write short note on the following:
Ammonolysis
