Advertisements
Advertisements
प्रश्न
Hydrogen peroxide is an oxidising agent. It oxidises ferrous ion to ferric ion and reduced itself to water. Write a balanced equation.
उत्तर
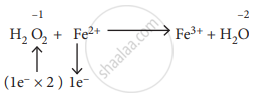
\[\ce{1H2O2 + 2Fe^2+ -> Fe^3+H2O}\]
\[\ce{H2O2 + 2Fe^2+ + 2H^+ -> 2Fe^3+ + 2H2O}\]
APPEARS IN
संबंधित प्रश्न
1 g of an impure sample of magnesium carbonate (containing no thermally decomposable impurities) on complete thermal decomposition gave 0.44 g of carbon dioxide gas. The percentage of impurity in the sample is
When 6.3 g of sodium bicarbonate is added to 30 g of acetic acid solution, the residual solution is found to weigh 33 g. The number of moles of carbon dioxide released in the reaction is
Total number of electrons present in 1.7 g of ammonia is
What do you understand by the term oxidation number?
Balance the following equation by the ion electron method.
\[\ce{C2O^2-_4 + Cr2O^2-_7 -> Cr^3+ + CO2}\] (in acid medium)
Balance the following equation by the ion electron method.
\[\ce{Na2S2O3 + I2 -> Na2S4O6 + NaI}\]
Reducing agent is a species that ____________.
Which of the following reagents is used for the following conversion?
\[\ce{CH3 - CH = CH - CHO -> CH3 - CH = CH - CH2OH}\]
In the redox reaction, \[\ce{2MnO^-_4 + SO^{2-}_3 + H2O -> 2MnO^{2-}_4 + SO^{2-}_4 + 2H^+}\], the reductant is:
In the cell represented by \[\ce{Pb(s) | Pb^{2+} (1 M) || Ag+ (1 M) | Ag(s)}\], the reducing agent is ______.
