Advertisements
Advertisements
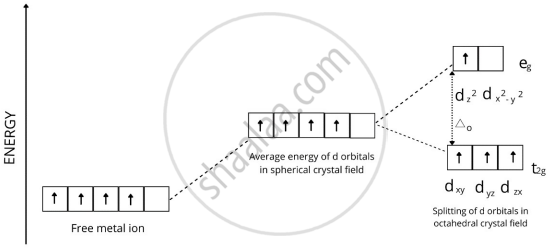
प्रश्न
In a coordination entity, the electronic configuration of the central metal ion is t2g3 eg1
Draw the crystal field splitting diagram for the above complex.
उत्तर
APPEARS IN
संबंधित प्रश्न
On the basis of crystal field theory, write the electronic configuration for d4 ion if Δ0 > P.
On the basis of crystal field theory, write the electronic configuration for d4 ion if ∆0 < P.
The hexaquo manganese (II) ion contains five unpaired electrons, while the hexacyanoion contains only one unpaired electron. Explain using Crystal Field Theory.
What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
On the basis of crystal field theory explain why Co(III) forms paramagnetic octahedral complex with weak field ligands whereas it forms diamagnetic octahedral complex with strong field ligands.
Why are low spin tetrahedral complexes not formed?
Using crystal field theory, draw energy level diagram, write electronic configuration of the central metal atom/ion and determine the magnetic moment value in the following:
\[\ce{[CoF6]^{3-}, [Co(H2O)6]^{2+}, [Co(Cn)6]^{3-}}\]
The CFSE for octahedral [CoCl6]−4 is 18,000 cm−1. What will be the CFSE for tetrahedral [CoCl3]−2?
[Ni(H2O)6]2+ (aq) is green in colour whereas [Ni(H2O)4 (en)]2+ (aq)is blue in colour, give reason in support of your answer.
What is the spectrochemical series?
