Advertisements
Advertisements
Question
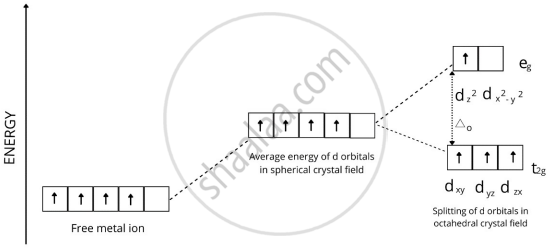
In a coordination entity, the electronic configuration of the central metal ion is t2g3 eg1
Draw the crystal field splitting diagram for the above complex.
Solution
APPEARS IN
RELATED QUESTIONS
Write the electronic configuration of Fe(III) on the basis of crystal field theory when it forms an octahedral complex in the presence of (i) strong field, and (ii) weak field ligand. (Atomic no.of Fe=26)
Complete and balance the following reactions:
(1) P4 + H2SO4 → ____ + _____ + _____ + _____
(2) Ag + HNO3(dilute) → _____ + ______ + _____ + _____
The CFSE for octahedral \[\ce{[CoCl6]^{4-}}\] is 18,000 cm–1. The CFSE for tetrahedral \[\ce{[CoCl4]^{2-}}\] will be ______.
Give the electronic configuration of the following complexes on the basis of Crystal Field Splitting theory.
\[\ce{[CoF6]^{3-}, [Fe(CN)6]^{4-} and [Cu(NH3)6]^{2+}}\].
Why are different colours observed in octahedral and tetrahedral complexes for the same metal and same ligands?
Considering crystal field theory, strong-field ligands such as CN–:
What is the difference between a weak field ligand and a strong field ligand?
For octahedral Mn(II) and tetrahedral Ni(II) complexes, consider the following statements:
(i) Both the complexes can be high spin.
(ii) Ni(II) complex can very rarely below spin.
(iii) With strong field Ligands, Mn(II) complexes can be low spin.
(iv) Aqueous solution of Mn (II) ions is yellow in colour.
The correct statements are:
Consider that d6 metal ion (M2+) forms a complex with aqua ligands and the spin only magnetic moment of the complex is 4.90 BM. The geometry and the crystal field stabilization energy of the complex is:
Explain the difference between a weak field ligand and a strong field ligand.
