Advertisements
Advertisements
प्रश्न
Mark the correct order of decreasing acid strength of the following compounds.
| (a) | 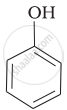 |
| (b) | 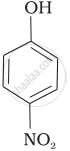 |
| (c) | 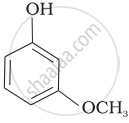 |
| (d) | 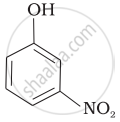 |
| (e) | 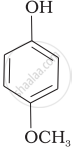 |
विकल्प
e > d > b > a > c
b > d > a > c > e
d > e > c > b > a
e > d > c > b > a
उत्तर
b > d > a > c > e
Explanation:
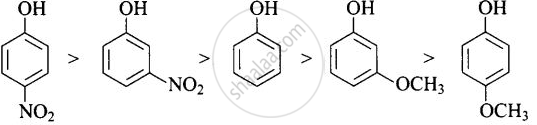
-NO2 is an electron-withdrawing group that increases the acidity of phenol and the effect is more pronounced at ortho and para positions. Similarly, the methoxy group is an electron releasing group that decreases the acidity of phenol and the effect is more pronounced at ortho and para positions.
APPEARS IN
संबंधित प्रश्न
Give the structure of the product you would expect when the following alcohol reacts with HCl–ZnCl2.
Butan-1-ol
Explain how does the −OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Acidity of phenol is due to ____________.
In CH3CH2OH, the bond that undergoes heterolytical change most readily is ____________.
Strength of acidity is in order:
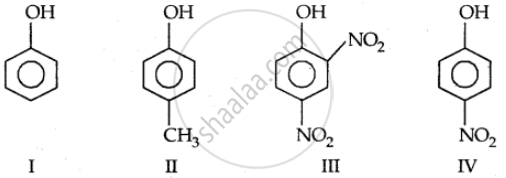
Which of the following compounds is most acidic?
Which of the following statements is true:
Out of o-nitrophenol and o-cresol which is more acidic?
Phenol is used in the manufacture of
Give the structure of the product you would expect when the following alcohol reacts with HCl–ZnCl2.
2-Methylbutan-2-ol
