Advertisements
Advertisements
प्रश्न
Match the columns.
| Group A | Group B |
| 1. ZnS | a) Copper Sulphide |
| 2. HgS | b) Bauxite |
| c) Cinnabar | |
| d) Zinc blend |
उत्तर
| Group A | Group B |
| 1. ZnS | d) Zinc blend |
| 2. HgS | c) Cinnabar |
APPEARS IN
संबंधित प्रश्न
Why are alkali metals kept in kerosene oil?
A student has been collecting silver coins and copper coins. One day she observed a black
coating on silver coins and a green coating on copper coins. Which chemical phenomenon is
responsible for these coatings? Write the names of black and green coatings.
Explain the following terms:
(a) flux
On which factors does the purification of metals depend?
Name the methods used for purification?
`ZnCO_3`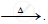 ..............................
..............................
`2Pb(NO3)_2`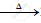 .........................
.........................
Give the ionization reactions of electrolyte used in Hall’s process. write the reaction at the cathode and the anode. Why the anode has to be replaced in this process?
Why is flux used in the blast furnace?
Distinguish between electrolytic methods of reduction and refining.
Aluminium is extracted from its chief ore, bauxite. The ore is first purified and then the metal is extracted from it by electrolytic reduction.
Write three balanced equations for the purification of bauxite.
Name the following:
Name an allotrope of a non-metal that allows electricity to pass through it.
Explain the hydraulic separation method with a neat labelled diagram.
State the reason for the addition of caustic alkali to bauxite ore during the purification of bauxite.
Zirconium is refined by ____________.
Which of following metals occurs in native state?
A process of extracting metals from aqueous solutions of their salts using suitable reducing agents is called ______
After partial roasting, the sulphide of copper is reduced by ______.
