Advertisements
Advertisements
प्रश्न
Mention the reaction condition and give balanced equation to obtain : HCl gas from common salt
उत्तर
\[\ce{\underset{\text{Sodium chloride}}{NaCl} + H2SO4 ->[<200°C] \underset{\text{Sodium hydrogen sulphate}}{NaHSO4} + HCl }\]
APPEARS IN
संबंधित प्रश्न
Certain blank spaces are left in the following table and these are labelled as A, B, C, D
and E. Identify each of them
| Lab preparation of | Reactants used | Products formed | Drying Agent | Method of collection |
|
| 1 | HCl gas | NaCl + H2SO4 |  |
conc. H2SO4 | 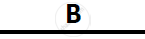 |
| 2 | NH3 gas |
|
Mg(OH)2 NH3 |
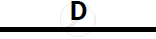 |
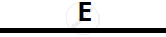 |
Identify the gas evolved and give the chemical test in the following cases
Dilute hydrochloric acid reacts with iron (II) sulphide.
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
Name the drying agent used and justify your choice.
State your observation in given case When dilute hydrochloric acid is added to sodium carbonate crystals
Give the balanced equation for the laboratory preparation of hydrogen chloride gas reaction.
Explain why hydrogen chloride gas is not collected over water.
How will you prove that the gas prepared is HCI?
Explain, why (or give reasons for)
Hydrogen chloride is not collected over water.
Describe an experiment to prove the following:
HCI gas contains the element chlorine.
What is aqua-regia?
Mention the reaction condition and give balanced equation to obtain: Cl2 gas from HCI gas.
Explain, why the following statement is not correct:
Lead chloride can be prepared by adding dilute HCI to lead sulphate solution.
Hydrogen chloride gas, being highly soluble in water, is dried by ______.
State the observation for action of dilute hydrochloiric acid or iron (II) sulphate.
Answer the following question related to the laboratory preparation of the hydrogen chloride gas:
Write the chemical equation.
In the laboratory preparation, HCl gas is dried by passing through ______.
