Advertisements
Advertisements
प्रश्न
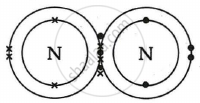
Show the covalent bond formation in nitrogen molecule.
उत्तर
APPEARS IN
संबंधित प्रश्न
Give reason why carbon compounds are generally poor conductors of electricity.
Ethane, with the molecular formula C2H6 has ______.
Fill in the blanks in the following sentence:
In forming oxygen molecule, .............. electrons are shared by each atom of oxygen.
Name one covalent compound containing chlorine.
Draw the electron-dot structure of HCl compound and state the type of bonding.
Draw the electron-dot structure of N2 and state the type of bonding.
Give the formulae of the chlorides of the elements X and Y having atomic numbers of 3 and 6 respectively. Will the properties of the two chlorides be similar or different? Explain your answer.
An element E exists in three allotropic forms A, B and C. In allotrope A, the atoms of element E are joined to form spherical molecules. In allotrope B, each atom of element E is surrounded by three other E atoms to form a sheet like structure. In allotrope C, each atom of element E is surrounded by four other E atoms to form a rigid structure.
(a) Name the element E.
(b) What is allotrope A.
(c) What is allotrope B?
(d) What is allotrope C?
(e) Which allotrope is used in making jewellery?
(f) Which allotrope is used in making anode of a dry cell?
Distinguish between ionic and covalent compounds under the following properties:
(i) Strength of forces between constituent elements
(ii) Solubility of compounds in water
(iii) Electrical conduction in substances
Explain the following term with example.
Homopolymer
Explain the following briefly:
Cl2 is a non polar molecule, while HCl is a polar molecule.
Give examples for the following:
Two solid covalent compounds.
Choose the correct answer from the options given below
Which one is not example of polar covalent compound?
Acids dissolve in water and produce positively charged ions. Draw the structure of these positive ions.
Elements Q and S react together to form an ionic compound. Under normal conditions, which physical state will the compound QS exist in?
Name greenish-yellow gas which also bleaches.
Write an Explanation.
Alkane
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
| \[\begin{array}{cc} \phantom{..}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\ \phantom{..}|\phantom{....}|\phantom{....}|\\ \ce{H - C - C- C- H}\\ \phantom{.}|\phantom{....}|\phantom{....}|\\ \ce{H - C - H}\\ |\\\ce{H}\end{array}\] |
The table shows the electronic structures of four elements.
| Element | Electronic Structure |
| P | 2, 6 |
| Q | 2, 8, 1 |
| R | 2, 8, 7 |
| S | 2, 8, 8 |
- Identify which element(s) will form covalent bonds with carbon.
- “Carbon reacts with an element in the above table to form several compounds.” Give suitable reason.
Which of the following compound(s) possesses a high melting point?
