Advertisements
Advertisements
प्रश्न
Solve the following.
At 0°C, a gas occupies 22.4 liters. How much hot must be the gas in celsius and in kelvin to reach a volume of 25.0 liters?
उत्तर
Given:
V1 = Initial volume of the gas = 22.4 L,
T1 = Initial temperature = 0 + 273.15 = 273.15 K,
V2 = Final volume = 25.0 L
To find: T2 = Final temperature in Celsius and in Kelvin
Formula: `"V"_1/"T"_1="V"_2/"T"_2` (at constant n and P)
Calculation:
According to Charles’ law,
`"V"_1/"T"_1="V"_2/"T"_2` (at constant n and P)
∴ T2 = `("V"_2xx"T"_1)/"V"_1`
∴ T2 = `(25.0xx273.15)/22.4` = 304.85 K ≈ 304.9 K
Converting to Celsius scale:
304.85 K = 304.85 − 273.15°C = 31.7°C
The temperature of the gas must be 31.7°C or 304.9 K
APPEARS IN
संबंधित प्रश्न
What would be the mass of CO2 occupying a volume of 44 litres at 25°C and 750 mm pressure.
State the following:
The absolute temperature of a gas at 7°C
Answer in one sentence.
A bubble of methane gas rises from the bottom of the North sea. What will happen to the size of the bubble as it rises to the surface?
Convert the following temperature from degree Celcius to kelvin.
−15° C
Convert the following temperature from degree Celcius to kelvin.
273° C
Convert the following pressure value into Pascals.
1 atmosphere
Convert 89 kPa to newton per square metre (Nm−2)
Convert 101.325 kPa to bar.
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
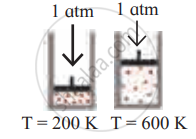 |
______________ |
With the help of the graph answer the following -

At constant temperature, Write the statement of law.
Solve the following.
A syringe has a volume of 10.0 cm3 at pressure 1 atm. If you plug the end so that no gas can escape and push the plunger down, what must be the final volume to change the pressure to 3.5 atm?

Solve the following.
The volume of a given mass of a gas at 0°C is 2 dm3. Calculate the new volume of the gas at constant pressure when the temperature is increased by 10°C.
Solve the following.
A hot air balloon has a volume of 2800 m3 at 99°C. What is the volume if the air cools to 80°C?

Name two items that can serve as a model for Gay Lusaac’s law and explain.
Explain the following observation.
Aerated water bottles are kept under water during summer
Sulphur hexafluoride is a colourless, odourless gas; calculate the pressure exerted by 1.82 moles of the gas in a steel vessel of volume 5.43 dm3 at 69.5 °C, assuming ideal gas behaviour
A small bubble rises from the bottom of a lake where the temperature and pressure are 6°C and 4 atm. to the water surface, where the temperature is 25°C and pressure is 1 atm. Calculate the final volume in (mL) of the bubble, if its initial volume is 1.5 mL.
Hydrochloric acid is treated with a metal to produce hydrogen gas. Suppose a student carries out this reaction and collects a volume of 154.4 × 10−3 dm3 of a gas at a pressure of 742 mm of Hg at a temperature of 298 K. What mass of hydrogen gas (in mg) did the student collect?
For a given mass of an ideal gas, which of the following statements is CORRECT?
A certain sample of gas has a volume of 0.2 L at one atmosphere pressure and 273.15 K. What is the volume of gas at 273.15°C at same pressure?
According to Andrews isothermals, the minimum temperature at which carbon dioxide gas obeys Boyles law is ______.
According to Andrews isothermals at what temperature the carbon dioxide gas starts to condense at 73 atmosphere?
At what temperature the volume of a gas becomes absolutely zero?
A gas occupies a volume of 4.2 dm3 at 101 kPa pressure. What volume will gas occupy if the pressure is increased to 235 kPa keeping the temperature constant?
If 2 moles of an ideal gas at 546 K has volume of 44.8 L, then what will be it's pressure? (R = 0.082)
The number of molecules in 8.96 litres of gas at 0°C and 1 atm. pressure is approximately ______.
