Advertisements
Advertisements
प्रश्न
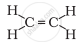
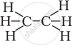
Structural formula of ethyne is
विकल्प
`"H" - "C" ≡ "C "- "H"`
`"H"_3-"C" ≡ "C" - "H"`
उत्तर
`"H" - "C" ≡ "C "- "H"`
The formula of ethyne is C2H2. There is one triple bond between carbon atoms.
APPEARS IN
संबंधित प्रश्न
Define Saturated hydrocarbon.
Why does carbon form strong bonds with most other elements ?
What will be the formula and electron dot structure of cyclopentane?
Why are certain compounds called hydrocarbons? Write the general formula for homologous series of alkanes, alkenes and alkynes and also draw the structure of the first member of each series. Write the name of the reaction that converts alkenes into alkanes and also write a chemical equation to show the necessary conditions for the reaction to occur.
Write the electron-dot structures for ethane.
Write the electron-dot structures for ethene.
How many isomers of the following hydrocharbons are possible?
C3H8
Fill in the blanks and rewrite the completed statements:
The organic compounds having double or triple bond in them are termed as _________________ _________________.
Which among the following are unsaturated hydrocarbons?
- \[\ce{H3C - CH2 - CH2 - CH3}\]
- \[\ce{H3C - C ≡ C - CH3}\]
- \[\begin{array}{cc}
\ce{H3C - CH - CH3}\\
|\phantom{..}\\
\ce{CH3}\\
\end{array}\] - \[\begin{array}{cc}
\ce{H3C - C = CH2}\\
|\\
\phantom{...}\ce{CH3}\\
\end{array}\]
Identify and name the functional groups present in the following compounds.
- \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H - C - C - C - OH}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
\end{array}\] - \[\begin{array}{cc}
\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{....}\\
\phantom{...}|\phantom{....}|\phantom{....}||\phantom{....}\\
\ce{H - C - C - C - OH}\\
\phantom{...}|\phantom{....}|\phantom{.........}\\
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{.......}\\
\end{array}\] - \[\begin{array}{cc}
\phantom{......}\ce{H}\phantom{....}\ce{H}\phantom{....}\ce{O}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{.......}\\
\phantom{....}|\phantom{....}|\phantom{....}||\phantom{....}|\phantom{....}|\phantom{....}\\
\ce{H} - \ce{C} - \ce{C} - \ce{C} - \ce{C} - \ce{C} - \ce{H}\\
\phantom{....}|\phantom{.....}|\phantom{........}|\phantom{....}|\phantom{....}\\
\phantom{....}\ce{H}\phantom{....}\ce{H}\phantom{........}\ce{H}\phantom{..}\ce{H}\phantom{.....}\\
\end{array}\] - \[\begin{array}{cc}
\phantom{......}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{......}\\
\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}\\
\ce{H - C - C - C = C - H}\\
\phantom{....}|\phantom{....}|\phantom{.............}\\
\phantom{....}\ce{H}\phantom{...}\ce{H}\phantom{.............}
\end{array}\]
