Advertisements
Advertisements
प्रश्न
The diagram below shows a cooling curve for a substance:
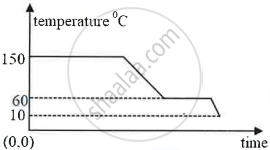
- State the temperatures at which the substance condenses.
- The temperature range in which the substance is in liquid state.
- Why do we prefer ice to ice-cold water for cooling a drink?
उत्तर
- The substance condenses at 150°C.
- 150°C to 60°C.
- At 0°C, melting 1 g of ice into water requires 336 J of heat energy from the drink. As a result, the drink releases 336 J more heat energy than 1 g ice at 0°C. As a result, 1 g of ice at 0°C produces significantly more cooling than 1 g of water at 0°C.
APPEARS IN
संबंधित प्रश्न
State two characteristics of a good thermion emitter.
- Which requires more heat: 1 g ice at 0℃ or 1 g water at 0℃ to raise its temperature to 10℃?
- Explain your answer in part (a).
A molten metal of mass 150 g is kept at its melting point 800℃. When it is allowed to freeze at the same temperature, it gives out 75,000 J of heat energy.
- What is the specific latent heat of the metal?
- If the specific heat capacity of metal is 200 J kg-1 K-1, how much additional heat energy will the metal give out in cooling to -50℃?
When a liquid is getting converted into solid, the latent heat is ………………………………
A substance changes from its solid state to the liquid state when heat is supplied to it. What name is given to heat absorbed by the substance.
What happens to the heat supplied to a substance when the heat supplied causes no change in the temperature of the substance?
Explain, why no tracks are left on the ice during ice skating?
Why does evaporation causes cooling and why is water used in hot water bottles?
If there is no Heat loss to the surroundings, the heat released by the condensation of m1 g of steam at 100°C into water at 100°C can be used to convert m2 g of ice at 0°C into water at 0°C.
(i) Find:
(a) The heat lost by steam in terms of m1
(b) The heat gained by ice in terms of m2
(ii) Form a heat equation find the ratio of m2 : m1
Specific latent heat of vaporization of steam = 2268 kJ/kg
Specific latent heat of fusion of ice = 336 kJ/kg
Specific heat capacity of water = 4200 J/kg°C
Find the odd one out and give its explanation.
Write the name.
The phase in which solid substances are converted into liquid.
During reheating, ice is converted to water at a temperature of 0 °C.
1 kg of dry air at a temperature of 40 °C can hold a maximum of 49 g of water vapour.
Write scientific reason.
Use a pressure cooker to cook food in cold air.
Who introduced the term latent heat?
Give some practical applications of specific latent heat of ice.
Calculate the amount of heat required to convert 200g of ice at 0°C into the water at 0°C Specific latent heat of fusion of ice = 336 Jg-1
The amount of heat energy required to melt a given mass of a substance at its melting point without any rise in its temperature is called as the ______.
