Advertisements
Advertisements
प्रश्न
उत्तर
APPEARS IN
संबंधित प्रश्न
State two characteristics of a good thermion emitter.
What do you understand by the term latent heat?
Explain the following:
The surrounding become pleasantly warm when water in a lake starts freezing in cold countries.
The S.I. unit of specific latent heat is ______.
The specific latent heat of fusion of water is ______.
During transformation of liquid phase to solid phase, the latent heat is ______.
Answer the following:
Explain the role of latent heat in the change of state of a substance.
What do you mean by the statement?
'The specific latent heat capacity of fusion of ice is 336 J per g'?
Calculate the total amount of heat required to convert 100g ice at 0°C to steam at 100°C.
(Specific latent heat of fusion of ice = 336 J/g, specific latent heat of vaporization of steam = 2260 J/g, specific heat capacity of water = 4.2 J/g°C).
Find the odd one out and give its explanation.
How fog is formed?
Match the columns.
| Column A | Column B |
| 1) Absolute humidity | a) J or cal |
| 2) Latent heat | b) J/kg °C |
| 3) Specific heat capacity | c) kJ/kg |
| 4) Heat | d) no unit |
| e) kg/m3 |
Write the name.
The phase in which solid substances are converted into liquid.
The latent heat of vaporisation is a term referred for the conversion of gas into liquid.
During reheating, ice is converted to water at a temperature of 0 °C.
1 kg of dry air at a temperature of 40 °C can hold a maximum of 49 g of water vapour.
Specific latent heat L = ______.
Specific latent heat of a substance ______.
The diagram below shows a cooling curve for a substance:
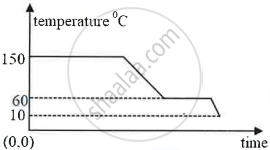
- State the temperatures at which the substance condenses.
- The temperature range in which the substance is in liquid state.
- Why do we prefer ice to ice-cold water for cooling a drink?
