Advertisements
Advertisements
प्रश्न
What happens to the heat supplied to a substance when the heat supplied causes no change in the temperature of the substance?
उत्तर
This heat supplied is used in the change of state. This heat is known as Latent heat.
APPEARS IN
संबंधित प्रश्न
State two characteristics of a good thermion emitter.
Explain the following:
The heat supplied to a substance during it change of state, does not cause any rise in its temperature.
The S.I. unit of specific latent heat is ______.
A refrigerator converts 100g of water at 20℃ to ice at – 10℃ in 73.5 min. Calculate the average rate of heat extraction in watt. The specific heat capacity of water is 4.2 J kg-1 K-1, specific latent heat of ice is 336 J g-1 and the specific heat capacity of ice is 2.1 J kg-1 K-1.
Explain the following temperature vs time graph.

What do you mean by the statement?
'The specific latent heat capacity of fusion of ice is 336 J per g'?
Define the following terms:
(i) Latent heat,
(ii) Latent heat of fusion of ice.
A substance changes from its solid state to the liquid state when heat is supplied to it. What name is given to heat absorbed by the substance.
Explain the meaning of the term latent heat. State its S. I. unit.
Define the term ‘specific latent heat of fusion’ of a substance.
1 kg of water is contained in a 1.25 kW kettle. Assuming specific heat capacity of water = 4.2 J/g °C and specific latent heat of vaporization = 2260 J/g, calculate:
(i) the time taken for the temperature of water to rise from 25°C to its boiling point,
(ii) the mass of water which evaporates per minute from the boiling water.
If pressure increases, the melting point of a substance ______.
Write the name.
Products obtained when sugar is heated.
Write the name.
The phase in which solid substances are converted into liquid.
During reheating, ice is converted to water at a temperature of 0 °C.
Specific latent heat L = ______.
2875 J of heat is required to melt 115 g of lead at its melting point. Calculate the specific latent heat capacity of fusion of lead.
Specific latent heat of a substance ______.
The diagram below shows a cooling curve for a substance:
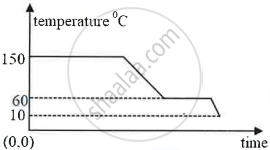
- State the temperatures at which the substance condenses.
- The temperature range in which the substance is in liquid state.
- Why do we prefer ice to ice-cold water for cooling a drink?
