Advertisements
Advertisements
प्रश्न
What happens when methanal undergoes cannizzaro reaction?
उत्तर १
When methanal (formaldehyde, HCHO) undergoes the Cannizzaro reaction, it results in the formation of formic acid HCOOH and methanol CH3OH.
The Cannizzaro reaction is a base-catalyzed disproportionation reaction that occurs with non-alpha-hydrogenated aldehydes (like methanal). In this reaction, one molecule of formaldehyde gets reduced to methanol CH3OH, while the other gets oxidized to formic acid HCOOH.
The reaction can be represented as:
\[\ce{2HCHO ->[NaOH] HCOOH + CH3OH}\]
उत्तर २
Methanal will undergo Cannizzaro reaction as it possesses α-hydrogen to form methanol.
\[\begin{array}{cc}
\phantom{.}\ce{H}\phantom{..............................}\ce{H}\phantom{.......}\ce{H}\phantom{...........}\phantom{..}\\
\backslash\phantom{..............................}|\phantom{.........}\backslash\phantom{..........}\\
\ce{2C = O + conc.KOH -> H - C - OH + C - O- K+}\\
/\phantom{..............................}|\phantom{.........}/\phantom{..........}\\
\ce{\underset{Methanal}{H}}\phantom{.........................}\ce{\underset{Methanol}{H}}\phantom{.}\ce{\underset{methanoate}{\underset{Potassium}{H}}}\phantom{............}\\
\end{array}\]
APPEARS IN
संबंधित प्रश्न
Complete the following reactions:
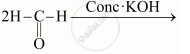
Describe the following:
Cannizzaro reaction
Write the reactions involved in the following reactions: Clemmensen reduction
Write the product(s) in the following reactions

Write the equations involved in the following reactions:
Etard reaction
Complete the following reactions:
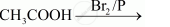
complete the following reaction:
\[\begin{array}{cc}
\phantom{...}\ce{CH3} \\
| \\
\phantom{.................}\ce{CH3-CH-COOH ->[(i) Br2/Red P4][(ii)H2O]}
\end{array}\]
Complete the following reaction:

Explain the following reaction:
Cannizzaro reaction
Convert the following:
Benzene to m-nitrobenzaldehyde
