Advertisements
Advertisements
प्रश्न
What is the coordination number in a square close packed structure in two dimensions?
विकल्प
2
3
4
6
उत्तर
4
Explanation:
Coordination number in a square closed packed structure in two dimensions is equal to 4 is shown as:
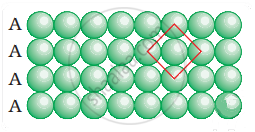
APPEARS IN
संबंधित प्रश्न
What is the two dimensional coordination number of a molecule in square close packed layer?
Aluminium crystallises in a cubic close-packed structure. Its metallic radius is 125 pm.
(i) What is the length of the side of the unit cell?
(ii) How many unit cells are there in 1.00 cm3 of aluminium?
The arrangement ABC ABC.... is referred to as ____________.
In the SF4 molecule, there are:
The octane number of Iso-octane is
Co-ordination number of sodium ion Na+ in Nacl is:-
The number of tetrahedral and octahedral voids in a CCP array of 100 atoms are respectively:
The right option for the number of tetrahedral and octahedral voids in the hexagonal primitive unit cell is _______.
Element 'B' forms ccp structures and A occupies half of the octahedral voids, while oxygen atoms occupy all the tetrahedral voids. The structure of bimetallic oxide is ______.
The density of a pure substance 'X' whose atoms pack in cubic close pack arrangement is 1 g/cc. If all tetrahedral voids are occupied by 'Y' atoms. The value of '3a' is ______ g/cc, if the density of resulting solid is 'a' g/cc.
[Given: Atomic mass (X) = 30 g/mol, (Y) = 20 g/mol]
