Advertisements
Advertisements
Question
What is the coordination number in a square close packed structure in two dimensions?
Options
2
3
4
6
Solution
4
Explanation:
Coordination number in a square closed packed structure in two dimensions is equal to 4 is shown as:
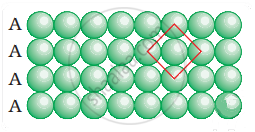
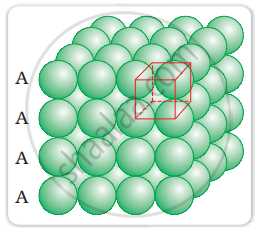
APPEARS IN
RELATED QUESTIONS
Ferric oxide crystallises in a hexagonal close-packed array of oxide ions with two out of every three octahedral holes occupied by ferric ions. Derive the formula of the ferric oxide.
Aluminium crystallises in a cubic close-packed structure. Its metallic radius is 125 pm.
(i) What is the length of the side of the unit cell?
(ii) How many unit cells are there in 1.00 cm3 of aluminium?
Which of the following statement is not true about the hexagonal close packing?
How can you best describe the elongated octahedral structure of blue vitriol, CuSO4.5H2O?
In the SF4 molecule, there are:
Total no. of voids in 0.5 mole of a compound forming hexagonal closed packed structure are:-
The packing efficiency of the two dimensional square unit cell shown below is:
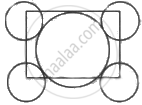
Which of the following is frenkal effect.
The right options for the number of tetrahedral and octahedral voids in the hexagonal primitive unit cells is ______.
The empirical formula for a compound with a cubic close packed arrangement of anions and with cations occupying all the octahedral sites in AxB. The value of x is ______. (Integer answer)
