Advertisements
Advertisements
Question
Which of the following statement is not true about the hexagonal close packing?
Options
The coordination number is 12.
It has 74% packing efficiency.
Tetrahedral voids of the second layer are covered by the spheres of the third layer.
In this arrangement spheres of the fourth layer are exactly aligned with those of the first layer.
Solution
In this arrangement spheres of the fourth layer are exactly aligned with those of the first layer.
Explanation:
Hexagonal close packing can be arranged by two layers
A and B one over another which can be diagrammatically represented as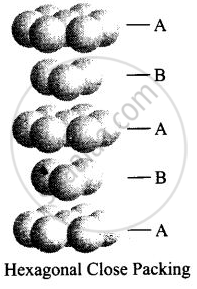
Here, we can see easily that 1st layer and 4th layer are not exactly aligned. Thus, statement (iv) is not correct while other statements (i), (ii) and (iii) are true.
APPEARS IN
RELATED QUESTIONS
What is the two dimensional coordination number of a molecule in square close packed layer?
A compound forms hexagonal close-packed structure. What is the total number of voids in 0.5 mol of it? How many of these are tetrahedral voids?
What is the coordination number in a square close packed structure in two dimensions?
In NaCl structure ____________.
The octane number of Iso-octane is
The crystal system of a compound with unit cell dimensions a = 0.387, b = 0.387 and c = 0.504 nm and α = β = 90° and ϒ = 120° is
A compound forms hexagonal close-packed structure. What is the total number of voids in 0.5 mol of it? How many of these are tetrahedral voids?
Which of the following is frenkal effect.
The right options for the number of tetrahedral and octahedral voids in the hexagonal primitive unit cells is ______.
The empirical formula for a compound with a cubic close packed arrangement of anions and with cations occupying all the octahedral sites in AxB. The value of x is ______. (Integer answer)
