Advertisements
Advertisements
प्रश्न
What is the correct order of reactivity of alcohols in the following reaction?
\[\ce{R-OH + HCl ->[ZnCl2] R-Cl + H2O}\]
विकल्प
1° > 2° > 3°
1° < 2° > 3°
3° > 2° > 1°
3° > 1° > 2°
उत्तर
3° > 2° > 1°
Explanation:
The given reaction is nucleophilic substitution reaction in which –OH group is replaced by –Cl. Tertiary alcohols, when react with \[\ce{HCl}\] in presence of \[\ce{ZnCl2}\], form tertiary carbocation.
This intermediate 3° carbocation is more stable than 2° carbocation as well as 1° carbocation. The higher the stability of intermediate, the higher will be the reactivity of reactant molecule.
So, the order of reactivity of alcohols in the given reaction is 3° > 2° > 1°.
APPEARS IN
संबंधित प्रश्न
Write the equation involved in the acetylation of Salicylic acid.
Give two reactions that show the acidic nature of phenol.
Explain how does the −OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Phenol reacts with Br2 in CS2 at low temperature to give ____________.
Which one of the following compounds has the most acid nature?
Arrange the following in decreasing order of acidic character:
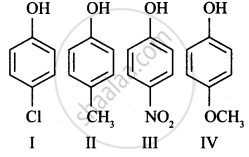
Which of the following statements is true:
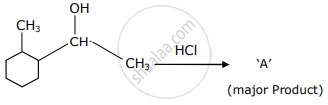
Which is the final product ‘A’ (major) in the given reaction?
For the pair phenol and cyclohexanol, answer the following:
Why is phenol more acidic than cyclohexanol?
Give the structure of the product you would expect when the following alcohol reacts with SOCl2.
2-Methylbutan-2-ol
