Advertisements
Advertisements
प्रश्न
For the pair phenol and cyclohexanol, answer the following:
Why is phenol more acidic than cyclohexanol?
उत्तर
Because the conjugate base of phenol, the phenoxide ion, is more stable due to resonance, phenol is more acidic than cyclohexanol.

APPEARS IN
संबंधित प्रश्न
Write the equation involved in the acetylation of Salicylic acid.
Account for the following:
o-nitrophenol is more steam volatile than p-nitrophenol.
Acidity of phenol is due to ____________.
In CH3CH2OH, the bond that undergoes heterolytical change most readily is ____________.
What is the correct order of reactivity of alcohols in the following reaction?
\[\ce{R-OH + HCl ->[ZnCl2] R-Cl + H2O}\]
Mark the correct order of decreasing acid strength of the following compounds.
| (a) | 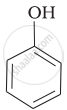 |
| (b) | 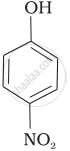 |
| (c) | 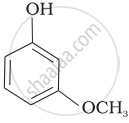 |
| (d) | 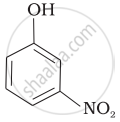 |
| (e) | 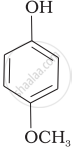 |
Phenol is used in the manufacture of
Give the structure of the product you would expect when the following alcohol reacts with HBr.
Butan-1-ol
Give the structure of the product you would expect when the following alcohol reacts with HBr.
2-Methylbutan-2-ol
Give the structure of the product you would expect when the following alcohol reacts with SOCl2.
Butan-1-ol
