Advertisements
Advertisements
प्रश्न
Which is the most suitable reagent for the following conversion?
\[\begin{array}{cc}
\phantom{....................}\ce{O}\phantom{.....................................}\ce{O}\phantom{.}\\
\phantom{....................}||\phantom{......................................}||\phantom{.}\\
\phantom{}\ce{CH3 - CH = CH - CH2 - C - CH3 -> CH3 - CH = CH - CH2 - C - OH}\phantom{.}
\end{array}\]
विकल्प
Tollen’s reagent
Benzoyl peroxide
\[\ce{I2}\] and \[\ce{NaOH}\] solution
\[\ce{Sn}\] and \[\ce{NaOH}\] solution
उत्तर
\[\ce{I2}\] and \[\ce{NaOH}\] solution
Explanation:
\[\begin{array}{cc}
\phantom{......................}\ce{O}\phantom{....................................}\ce{O}\phantom{..............}\\
\phantom{....................}||\phantom{.....................................}||\phantom{............}\\
\phantom{}\ce{CH3CH = CH - CH2 - C - CH3 ->[I2 + NaOH] CH3CH = CHCH2 - C - ONa + CHI3}\\
\phantom{............................................}\ce{↓ H2O}\\
\phantom{.......................................................}\ce{O}\\
\phantom{.......................................................}||\\
\phantom{.........................................}\ce{CH3CH = CHCH2 - C - OH}
\end{array}\]
APPEARS IN
संबंधित प्रश्न
Predict the products of the following reactions:
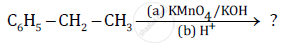
Name the reagents used in the following reactions:

Show how the following compound can be converted to benzoic acid.
Acetophenone
Show how the following compound can be converted to benzoic acid.
Phenylethene (Styrene)
An organic compound (A) (molecular formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-1-ene. Write equations for the reactions involved.
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
m-Nitrobenzoic acid
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
p-Nitrobenzoic acid
How will you bring about the following conversion in not more than two steps?
Benazaldehyde to α-Hydroxyphenylacetic acid
How is methoxy benzene prepared from carbolic acid?
Match the reactions given in Column I with the suitable reagents given in Column II.
| Column I (Reactions) |
Column II (Reagents) |
| (i) Benzophenone Diphenylmethane | (a) \[\ce{LiAlH4}\] |
| (ii) Benzaldehyde 1-Phenylethanol | (b) \[\ce{DIBAL-H}\] |
| (iii) Cyclohexanone Cyclohexanol | (c) \[\ce{Zn(Hg)/Conc. HCl}\] |
| (iv) Phenyl benzoate Benzaldehyde | (d) \[\ce{CH3MgBr}\] |
