Advertisements
Advertisements
प्रश्न
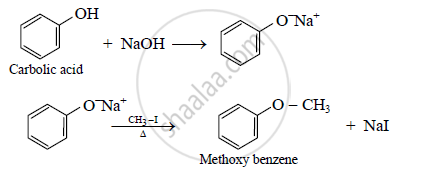
How is methoxy benzene prepared from carbolic acid?
उत्तर
APPEARS IN
संबंधित प्रश्न
Identify ‘A' and ‘B’ in the following reaction :
C6H5MgBr + C02 `(`> ‘A’ `(PCl_5)/()`> ‘B’
Predict the products of the following reactions:
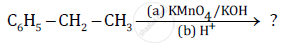
Name the reagents used in the following reactions:

Show how the following compound can be converted to benzoic acid.
Ethylbenzene
Show how the following compound can be converted to benzoic acid.
Acetophenone
Show how the following compound can be converted to benzoic acid.
Phenylethene (Styrene)
Predict the products formed when cyclohexanecarbaldehyde reacts with the following reagent.
Tollens’ reagent
An organic compound (A) (molecular formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-1-ene. Write equations for the reactions involved.
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
Methyl benzoate
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
m-Nitrobenzoic acid
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
p-Nitrobenzoic acid
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
Phenylacetic acid
How will you bring about the following conversion in not more than two steps?
Benazaldehyde to α-Hydroxyphenylacetic acid
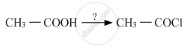
Complete the synthesis by giving missing starting material, reagent or product.
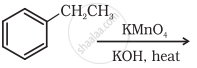
Name the reagents used in the following reactions:
What is the action of following reagents on glucose?
1. Bromine water
2. Hydroxylamine
The functional group present in triacylglycerol is _______.
The reagent which does not react with both, acetone and benzaldehyde.
Through which of the following reactions number of carbon atoms can be increased in the chain?
(i) Grignard reaction
(ii) Cannizaro’s reaction
(iii) Aldol condensation
(iv) HVZ reaction
Match the reactions given in Column I with the suitable reagents given in Column II.
| Column I (Reactions) |
Column II (Reagents) |
| (i) Benzophenone Diphenylmethane | (a) \[\ce{LiAlH4}\] |
| (ii) Benzaldehyde 1-Phenylethanol | (b) \[\ce{DIBAL-H}\] |
| (iii) Cyclohexanone Cyclohexanol | (c) \[\ce{Zn(Hg)/Conc. HCl}\] |
| (iv) Phenyl benzoate Benzaldehyde | (d) \[\ce{CH3MgBr}\] |
Substitution of one alkyl group by replacing hydrogen of primary amines
Benzoic acid can be obtained by the oxidation of all of the following EXCEPT ______.
Match List - I with List - II.
| List - I | List - II | ||
| (a) |  \[\ce{->[CO,HCl][Anhyd. AlCl3/CuCl]}\] \[\ce{->[CO,HCl][Anhyd. AlCl3/CuCl]}\] |
(i) | Hell-Volhard-Zelinsky reaction |
| (b) |
\[\begin{array}{cc}
\ce{O}\phantom{.................}\\ ||\phantom{.................}\\ \ce{R - C - CH3 + NaOX ->} \end{array}\] |
(ii) | Gattermann-Koch reaction |
| (c) | \[\ce{R - CH2 - OH + R'COOH ->[Conc. H2SO4]}\] | (iii) | Haloform reaction |
| (d) | \[\ce{R - CH2COOH ->[(i) X2/Red P][(ii) H2O]}\] | (iv) | Esterification |
Choose the correct answer from the options given below.
Alkaline hydrolysis of C4H8Cl2 gives a compound (A) which on heating with NaOH and I2 produces a yellow precipitate of CHI3. The compound (A) should be ______.
A compound 'X' with molecular formula C3H8O can be oxidised to a compound 'Y' with the molecular formula C3H6O2 'X' is most likely to be ______.
