Advertisements
Advertisements
प्रश्न
The functional group present in triacylglycerol is _______.
विकल्प
alcohol
ether
ester
amine
उत्तर
The functional group present in triacylglycerol is ester.
Triacylglycerol is a triester of glycerol with higher fatty acids.
APPEARS IN
संबंधित प्रश्न
On acid hydrolysis, propane nitrile gives.
How is carbolic acid prepared from chlorobenzene?
Write the structures of A and B in the following reactions
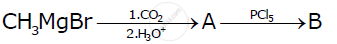
Predict the products of the following reactions:
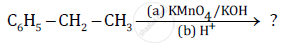
Name the reagents used in the following reactions:

Show how the following compound can be converted to benzoic acid.
Ethylbenzene
Show how the following compound can be converted to benzoic acid.
Acetophenone
Show how the following compound can be converted to benzoic acid.
Bromobenzene
Show how the following compound can be converted to benzoic acid.
Phenylethene (Styrene)
Predict the products formed when cyclohexanecarbaldehyde reacts with the following reagent.
Tollens’ reagent
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
Phenylacetic acid
How is methoxy benzene prepared from carbolic acid?
What is the action of following reagents on glucose?
1. Bromine water
2. Hydroxylamine
Which is the most suitable reagent for the following conversion?
\[\begin{array}{cc}
\phantom{....................}\ce{O}\phantom{.....................................}\ce{O}\phantom{.}\\
\phantom{....................}||\phantom{......................................}||\phantom{.}\\
\phantom{}\ce{CH3 - CH = CH - CH2 - C - CH3 -> CH3 - CH = CH - CH2 - C - OH}\phantom{.}
\end{array}\]
Through which of the following reactions number of carbon atoms can be increased in the chain?
(i) Grignard reaction
(ii) Cannizaro’s reaction
(iii) Aldol condensation
(iv) HVZ reaction
Match the reactions given in Column I with the suitable reagents given in Column II.
| Column I (Reactions) |
Column II (Reagents) |
| (i) Benzophenone Diphenylmethane | (a) \[\ce{LiAlH4}\] |
| (ii) Benzaldehyde 1-Phenylethanol | (b) \[\ce{DIBAL-H}\] |
| (iii) Cyclohexanone Cyclohexanol | (c) \[\ce{Zn(Hg)/Conc. HCl}\] |
| (iv) Phenyl benzoate Benzaldehyde | (d) \[\ce{CH3MgBr}\] |
Assertion: Aldehydes and ketones, both react with Tollen’s reagent to form silver mirror.
Reason: Both, aldehydes and ketones contain a carbonyl group.
A compound 'X' with molecular formula C3H8O can be oxidised to a compound 'Y' with the molecular formula C3H6O2 'X' is most likely to be ______.
Fill in the blanks by choosing the appropriate words from those given in the brackets:
[stable, low, aldehyde, unstable, 6, 4, ethane, Clemmensen’s, 2, 3, carboxylic acid, high, propane, Rosenmund's]
The primary alcohols are easily oxidised first into ______ and then into ______.
