Advertisements
Advertisements
प्रश्न
Why do we add alum to purify water?
उत्तर
River water carries negatively charged colloidal particles of clay and sand; alum \[\ce{(K2SO4Al2(SO4)3 24H2O)}\] is added to river water to coagulate the colloidal particles of clay and sand. Thus, alum helps to purify water.
APPEARS IN
संबंधित प्रश्न
Define the following term:
Zeta potential
Out of MgCl2 and AlCl3, which one is more effective in causing coagulation of negatively charged sol and why?
What modification can you suggest in the Hardy-Schulze law?
Explain what is observed When a beam of light is passed through a colloidal sol.
Explain the terms Dialysis
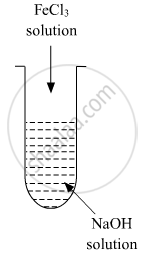
A colloidal sol is prepared by the given method in the figure. What is the charge on hydrated ferric oxide colloidal particles formed in the test tube? How is the sol represented?
The values of colligative properties of colloidal solution are of small order in comparison to those shown by true solutions of same concentration because of colloidal particles ______.
Match the statement given in Column I with the phenomenon given in Column II.
| Column I | Column II |
| (i) Dispersion medium moves in an electric field | (a) Osmosis |
| (ii) Solvent molecules pass through semi permeable membrane towards solvent side |
(b) Electrophoresis |
| (iii) Movement of charged colloidal particles under the influence of applied electric potential towards oppositely charged electrodes |
(c) Electroosmosis |
| (iv) Solvent molecules pass through semi permeable membranes towards solution side |
(d) Reverse osmosis |
What is Gold number?
Coagulation value of the electrolytes AlCl3 and NaCl for As2S3 sol are 0.093 and 52 respectively. The No. of times AlCl3 has greater coagulating power than NaCl is ______.
