Advertisements
Advertisements
प्रश्न
Why is \[\ce{NH2}\] group of aniline acetylated before carrying out nitration?
उत्तर
Direct nitration of aniline is not possible on account of oxidation of –NH2 group. However, nitration can be carried after protecting the –NH2 group by acetylation to give acetanilide which is then nitrated and finally hydrolysed to give o- and p-nitroanilines.
The acetyl group being electron-withdrawing attracts the lone pair of electrons of the N-atom towards carbonyl group.
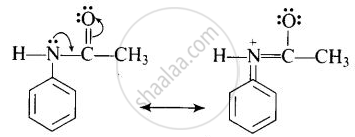
As a result, the activating effect –NH2 group is reduced i.e., the lone pair of electrons on nitrogen is less available for donation to benzene ring by resonance. Therefore, activating effect of –NHCOCH3 group is less than that of the –NH2 group.
APPEARS IN
संबंधित प्रश्न
Write a short note on diazotisation.
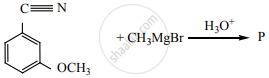
Product ‘P’ in the above reaction is:
Ammonium salt of benzoic acid is heated strongly with P2O5 and the product so formed is reduced and then treated with NaNO2/HCl at low temperature. The final compound formed is ____________.
The major product of the following reaction:
Write a short note on the following.
Coupling reaction
Account for the following.
Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Account for the following.
Amines are more basic than amides.
Identify A, B, C and D.
\[\ce{aniline + benzaldehyde -> A ->[Conc. HNO3][B] C + D}\]
What would be the major product of the following reaction?
\[\ce{C6H5 - CH2 - OC6H5 + HBr -> A + B}\]
Coupling of benzene diazonium chloride with 1-naphthol in alkaline medium will give:
