Advertisements
Advertisements
प्रश्न
Write the balanced chemical equation for the following and identify the type of reaction.
\[\ce{Zinc carbonate(s) -> Zinc oxide(s) + Carbon dioxide(g)}\]
उत्तर
\[\ce{ZnCO3(s) -> ZnO(s) + CO2(g)}\]
Type: Decomposition reaction
APPEARS IN
संबंधित प्रश्न
A solid substance P which is very hard is used in the construction of many buildings, especially flooring. When substance P is heated strongly, it decomposes to form another solid Q and a gas R is given out. Solid Q reacts with water with the release of a lot of heat to form a substance S. When gas R is passed into a clear solution of substance S, then a white precipitate of substance T is formed. The substance T has the same chemical composition as starting substance P.
(a) What is substance P? Write its common name as well as chemical formula.
(b) What is substance Q?
(c) What is gas R?
(d) What is substance S? What is its clear solution known as?
(e) What is substance T? Name any two natural forms in which substance T occurs in nature.
What type of chemical reaction take place when lime-stone is heated?
State the change in colour observed in following case mentioning the reason:
Silver chloride is exposed to sunlight.
Which of the following can be decomposed by the action of light?
(a) NaCl
(b) KCl
(c) AgCl
(d) CuCl
When a green iron salt is heated strongly, its colour finally changes to brown and odour of burning sulphur is given out.
(a) Name the iron salt.
(b) Name the type of reaction that takes place during the heating of iron salt.
(c) Write a chemical equation for the reaction involved.
A metal salt MX when exposed to light splits up to form metal M and a gas X2. Metal M is used in making ornaments whereas gas X2 is used in making bleaching powder. The salt MX is itself used in black and white photography.
(a) What do you think metal M is?
(b) What could be gas X2?
(c) Name the metal salt MX.
(d) Name any two salt solutions which on mixing together can produce a precipitate of salt MX.
(e) What type of chemical reaction takes place when salt MX is exposed to light? Write the equation of the reaction?
What do you mean by redox reaction ? Explain with the help of an example.
Study the following figure and answer questions.

a) After heating Calcium carbonate, which gas is formed in a test tube?
b) When we pass this gas through limewater what change, did you observe?
c) Write down the chemical reaction showing the product formation after heating the Calcium carbonate.
A student wants to study a decomposition reaction by taking ferrous sulphate crystals. Write two precautions he must observe while performing the experiment.
Complete the statement by filling in the blank with the correct word:
Decomposition of silver salts in the presence of sunlight is an example of _________.
Give a balanced equation for the following type of reaction:
A thermal decomposition reaction in which a compound decomposes to give two new compounds.
Differentiate between the following:
Electrolytic decomposition and photochemical decomposition.
Explain the reaction given in the figure.

Chemical volcano is an example of ______ type of reaction.
Which among the following statement(s) is (are) true?
Exposure of silver chloride to sunlight for a long duration turns grey due to
(i) the formation of silver by decomposition of silver chloride.
(ii) sublimation of silver chloride.
(iii) decomposition of chlorine gas from silver chloride.
(iv) oxidation of silver chloride.
Which of the following is an endothermic process?
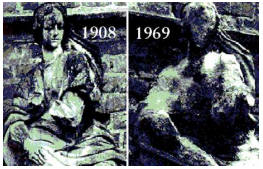
Marble statues are corroded or stained rain water. Identify the main reason.
Balance the following chemical equation and identify the type of chemical reaction.
`"HgO"("s") overset("(heat)")(->) "Hg"("l") + "O"_2("g")`
Balance the following chemical equation and identify the type of chemical reaction.
`"H"_2"O"_2("l") overset("U V")(->) "H"_2"O"("l") + "O"_2("g")`
