Advertisements
Advertisements
प्रश्न
Study the following figure and answer questions.

a) After heating Calcium carbonate, which gas is formed in a test tube?
b) When we pass this gas through limewater what change, did you observe?
c) Write down the chemical reaction showing the product formation after heating the Calcium carbonate.
उत्तर
a) carbon dioxide
b) lime water turns milky. 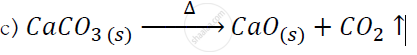
APPEARS IN
संबंधित प्रश्न
Taking into consideration the relationship in the first pair, complete the second pair
2H2 + O2 → 2H2O :Combination Reaction :: 2HgO → 2Hg + O2:_________
A solid substance P which is very hard is used in the construction of many buildings, especially flooring. When substance P is heated strongly, it decomposes to form another solid Q and a gas R is given out. Solid Q reacts with water with the release of a lot of heat to form a substance S. When gas R is passed into a clear solution of substance S, then a white precipitate of substance T is formed. The substance T has the same chemical composition as starting substance P.
(a) What is substance P? Write its common name as well as chemical formula.
(b) What is substance Q?
(c) What is gas R?
(d) What is substance S? What is its clear solution known as?
(e) What is substance T? Name any two natural forms in which substance T occurs in nature.
Give one example of a decomposition reaction which is carried out by applying heat.
Give one example of a decomposition reaction which is carried out with electricity.
What type of reaction is represented by the following equation?
2 FeSO4 → Fe2 O3 + SO2 + SO3
What type of chemical reaction take place when lime-stone is heated?
What type of chemical reaction is represented by the following equation?
X → Y + Z
What type of reaction is represented by the following equation?
NH4NO2 → N2 + 2H2O
Which of the following can be decomposed by the action of light?
(a) NaCl
(b) KCl
(c) AgCl
(d) CuCl
When a green iron salt is heated strongly, its colour finally changes to brown and odour of burning sulphur is given out.
(a) Name the iron salt.
(b) Name the type of reaction that takes place during the heating of iron salt.
(c) Write a chemical equation for the reaction involved.
A metal salt MX when exposed to light splits up to form metal M and a gas X2. Metal M is used in making ornaments whereas gas X2 is used in making bleaching powder. The salt MX is itself used in black and white photography.
(a) What do you think metal M is?
(b) What could be gas X2?
(c) Name the metal salt MX.
(d) Name any two salt solutions which on mixing together can produce a precipitate of salt MX.
(e) What type of chemical reaction takes place when salt MX is exposed to light? Write the equation of the reaction?
Explain the following type of chemical reaction, giving two examples for it:
Decomposition reaction
What are thermal decomposition reactions ? Explain with an example.
Give scientific reason.
When the gas formed on heating limestone, is passed through freshly prepared lime water, the lime water turns milky.
Identify the type of following reaction :
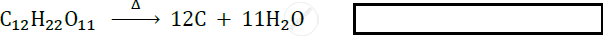
Classify the following reaction into different type:
\[\ce{2KClO3(s)->[\Delta] 2KCl(aq) + 3O2(g)}\]
Differentiate between direct combination reaction and a decomposition reaction.
Give a balanced equation for the following type of reaction:
A thermal decomposition reaction in which a metallic nitrate decomposes to give – a basic oxide.
Give a balanced equation for the following type of reaction:
A thermal decomposition reaction in which a compound decomposes to give two new compounds.
Differentiate between the following:
Electrolytic decomposition and photochemical decomposition.
What is electrolysis?
Explain the reaction given in the figure.

Photolysis is a decomposition reaction caused by ______
Chemical volcano is an example of ______ type of reaction.
Which one of the following processes involves chemical reactions?
Give the ratio in which hydrogen and oxygen are present in water by volume.
Which among the following statement(s) is (are) true?
Exposure of silver chloride to sunlight for a long duration turns grey due to
(i) the formation of silver by decomposition of silver chloride.
(ii) sublimation of silver chloride.
(iii) decomposition of chlorine gas from silver chloride.
(iv) oxidation of silver chloride.
When SO2 gas is passed through a saturated solution of H2S, which of the following reaction occurs?
Balance the following chemical equation and identify the type of chemical reaction.
`"H"_2"O"_2("l") overset("U V")(->) "H"_2"O"("l") + "O"_2("g")`
Complete the following reaction:
\[\ce{C_12H_22O11->[Heat]}\] ______ + ______.
A metal nitrate 'A' on heating gives a metal oxide along with evolution of a brown coloured gas 'B' and a colourless gas, which helps in burning. Aqueous solution of 'A' when reacted with potassium iodide forms a yellow precipitate.
- Identify 'A' and 'B'
- Name the types of the reactions involved in the above statement.
When lead nitrate is heated strongly in a boiling tube, two gases are liberated and a solid residue is left behind in the test tube.
- Name the type of chemical reaction and define it.
- Write the name and formula of the coloured gas liberated.
- Write the balanced chemical equation for the reaction.
- Name the residue left in the test tube and state the method of testing its nature (acidic/basic).
Write one equation for decomposition reactions where energy is supplied in the form of light.
Write one equation for decomposition reactions where energy is supplied in the form of electricity.
