SSC (English Medium)
SSC (Marathi Semi-English)
Academic Year: 2018-2019
Date: मार्च 2019
Advertisements
For freely falling object we can write the Newton’s second equation of motion as …..
Chapter: [0.01] Gravitation
Molecular formula of the chloride of an element X is XCl. This compound is a solid having high melting point. Write name of any element present in the same group as X
Chapter: [0.08] Metallurgy
When heat energy is absorbed by object ΔT represents the raise in temperature. What would be ΔT represent if the object loses heat energy?
Chapter: [0.05] Heat
Which is the non-ionic compound in the compounds given below.
AgNO3 + Nacl → AgCl + NaNO3
Chapter: [0.08] Metallurgy
Observe the following figure which bulb get fuse?
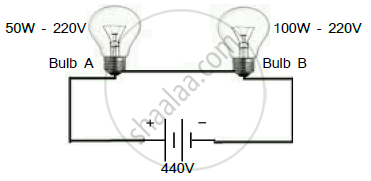
Chapter: [0.04] Effects of Electric Current [0.14] The Electric Spark
Yesh find out F1 and F2 of symmetric convex lens experimentally then which conclusion is true.
a) 𝐹1 = 𝐹2
b) 𝐹1 > 𝐹2
c) 𝐹1 < 𝐹2
d) 𝐹1 ≠ 𝐹2
Chapter: [0.07] Lenses [0.16] Wonders of Light 1
Choose and write the correct option.
If we gradually increase the angle of incidence of a ray of light passing through prism then …..
Angle of deviation goes on decreasing.
Angle of deviation decreases but after certain value of incident angle, deviation angle increases
Angle of deviation goes on increasing.
Angle of deviation increases but after certain value of incident angle deviation angle decreases.
Chapter: [0.06] Refraction of Light [0.17] Wonders of Light 2
Advertisements
Choose and write the correct option.
Which type of carbon-carbon bonds are present in Vanaspati ghee?
a) Single
b) double
c) triple
d) single-double
Chapter:
Choose and write the correct option.
What is the type of the following reaction?
BaCl2 + ZnSO4 → BaSO4 + ZnCl2
Displacement
combination
decomposition
double displacement
Chapter: [0.03] Chemical Reactions and Equations [0.12] The Magic of Chemical Reactions
Choose and write the correct option.
Which of the following astronauts travelled through space shuttle ‘Discovery’ first time?
a) Kalpana Chawala
b) Rakesh Sharma
c) Sunita Williams
d) Neil Armstrong
Chapter: [0.1] Space Missions
Which of this element belong to the period 3? Write their electronic configuration
`3^(Li) , 14^(Si) , 2^(He) , 15^(P)`
Chapter: [0.02] Periodic Classification of Elements
If in a medium, the speed of light is 1.5 × 108 m/s how much will the absolute refractive index of that medium be?
Chapter: [0.06] Refraction of Light [0.17] Wonders of Light 2
Prove the statement.
If the angle of incidence and angle of emergence of a light ray falling on a glass slab are i and e respectively, prove that, i = e.
Chapter: [0.06] Refraction of Light [0.17] Wonders of Light 2
At which position will you keep an object in front of convex lens to get a real image smaller than the object? Draw a figure.
Chapter: [0.07] Lenses [0.16] Wonders of Light 1
Complete the following flow chart.

Chapter: [0.09] Carbon Compounds
Why are geostationary satellites not useful for studies of polar regions?
Chapter: [0.1] Space Missions
Advertisements
Write the proper name of the orbits of satellites shown in the following figure with their height from the earth’s surface.

Chapter: [0.1] Space Missions
The radius of planet A is half the radius of planet B. If the mass of A is MA, what must be the mass of B so that the value of g on B is half that of its value on A?
Chapter: [0.01] Gravitation
Study the radius of the element given below and answer the following questions.
| elements | K | Na | Rb | Cs | Li |
| Atomic radius (pm) | 231 | 186 | 244 | 262 | 151 |
a) Which of the above elements have the smallest atom?
b) In which group of the modern periodic table the above element are belongs?
c) What is the periodic trend observed in the variation of atomic radii down a group?
Chapter: [0.02] Periodic Classification of Elements
Study the following figure and answer questions.

a) After heating Calcium carbonate, which gas is formed in a test tube?
b) When we pass this gas through limewater what change, did you observe?
c) Write down the chemical reaction showing the product formation after heating the Calcium carbonate.
Chapter: [0.03] Chemical Reactions and Equations [0.12] The Magic of Chemical Reactions
Give scientific reasons.
While preparing dil. Sulphuric acid from concentrated conc. sulphuric acid in the laboratory the concentrated sulphuric acid is, add slowly to water with constant stirring.
Chapter: [0.03] Chemical Reactions and Equations
Write the IUPAC names of the following structural formulae.
i. CH3- CH2- CH2- CH3
ii. CH3- CH2- COOH
iii. CH3 – CO- CH2- CH3
Chapter:
Explain the following temperature vs time graph.
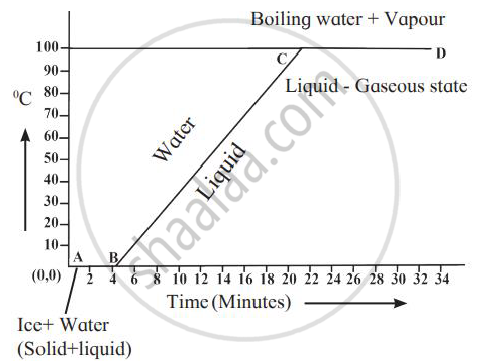
Chapter: [0.05] Heat
Observer the following diagram and answer the questions.
a) Which eye defect is shown in this diagram?
b) What are the possible reasons for this eye defect?
c) How this defect is corrected, write it in brief?
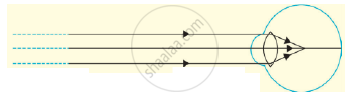
Chapter: [0.07] Lenses [0.16] Wonders of Light 1
Study the following principle and answer the question.
A force is excreted on the current carrying conductor. The direction of this force depends on both the direction of the current and the direction of the magnetic field. This force is maximum when the direction of current is perpendicular to the direction of the magnetic field.
a) By which law we can determine the direction of force excreted on the current carrying conductor.
b) In which electrical equipment this principle is used.
c) Draw a diagram representing construction of this equipment.
d) Write the working of this equipment in brief.
Chapter: [0.04] Effects of Electric Current [0.15] All about Electromagnetism
Answer the following question.
a) What is meant by corrosion?
b) Write names of any two methods of prevention of corrosion.
c) In which method, metal like copper, aluminium are coated with a thin layer of their oxides by means of electrolysis.
d) Explain this method with diagram.
Chapter: [0.03] Chemical Reactions and Equations [0.08] Metallurgy
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
Maharashtra State Board previous year question papers 10th Standard Board Exam Science and Technology 1 with solutions 2018 - 2019
Previous year Question paper for Maharashtra State Board 10th Standard Board Exam -2019 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science and Technology 1, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of Maharashtra State Board 10th Standard Board Exam.
How Maharashtra State Board 10th Standard Board Exam Question Paper solutions Help Students ?
• Question paper solutions for Science and Technology 1 will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
