Advertisements
Advertisements
प्रश्न
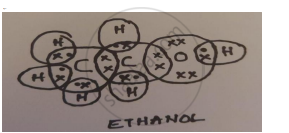
A carbon compound ‘A’ having melting point 156K and boiling point 351K, with molecular formula C2H6O is soluble in water in all proportions.
- Identify ‘A’ and draw its electron dot structure.
- Give the molecular formulae of any two homologues of ‘A’.
उत्तर
- Ethanol; C2H5OH
- CH3OH and C3H7OH are homologues of ethanol
OR
CH4O and C₃H₈O
APPEARS IN
संबंधित प्रश्न
Classify the following carbon compounds into two homologous series and name them.
C3H4, C3H6, C4H6, C4H8, C5H8, C5H10
Write the name and formula of the 2nd member of homologous series having general formula CnH2n.
Write the molecular formula of two consecutive members of homologous series of aldehydes. State which part of these compounds determines their
- physical and
- chemical properties
By how many carbon atoms and hydrogen atoms do any two adjacent homologues differ?
An organic compound having the molecular formula C3H6O can exist in the form of two isomers A and B having different functional groups. The isomer A is a liquid which is used as a solvent for nail polish. The isomer B is also a liquid. An aqueous solution of one of the lower homologues of B is used for preserving biological specimens in the laboratory
(a) What is compound A?
(b) Write the electron-dot structure of A.
(c) What is compound B?
(d) Write the electron-dot structure of B.
(e) Name the lower homologue of compound B which is used in preserving biological specimens.
What is the difference in the molecular formula of any two adjacent homologues in terms of molecular mass?
The general molecular formula for the homologous series of alkynes is _______.
Write a short note.
Homologous series
Which of the following pairs can be the successive members of a homologous series?
Which of the following does not belong to the same homologous series?
